Changes in plasma proteins in trauma, infection and inflammation – ‘the acute-phase response’
Following trauma, infection, inflammation, burns, etc., the body responds by initiating a series of mechanisms that lead to:
- Acute haemodynamic changes.
- A rapid fall in the concentration of some plasma proteins (e.g. albumin, transferrin).
- An increase in the concentration of several specific proteins some hours after the injury. These proteins are the acute-phase proteins, and are listed in Table 16.2.
The cytokines and a host of vasoactive substances are important mediators of the acute-phase response. The rapid decrease in concentration of certain proteins appears to result from loss of plasma protein into the extravascular space, due to increased vascular permeability caused by vasoactive substances, including cytokines, prostaglandins and histamine. The increase in the acute-phase plasma proteins results from increased synthesis and release, which appears to be mediated by interleukin-6.
Table 16.1 Examples of plasma proteins commonly measured for the diagnosis and monitoring of disease
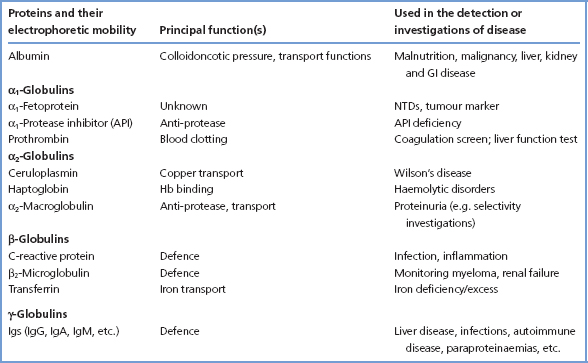
Figure 16.1 Separation of serum proteins by electrophoresis. The upper figure (a) shows the pattern in a normal serum sample. The lower figure (b) shows the typical discrete band found in the γ-globulin region in patients with myeloma.
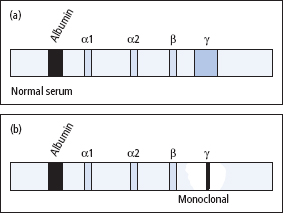
Table 16.2 Examples of acute-phase proteins that increase

Table 16.3 Causes of hypoalbuminaemia
| Artefact | Physiological | Pathological |
| Dilution of sample – sample taken from drip arm | Pregnancy | Impaired synthesis, malnutrition, malabsorption, chronic liver disease, analbuminaemia |
| Recumbency | Excessive loss from kidney, GI tract or skin Overhydration | |
| Increased capillary permeability – hypoxia, sepsis, etc. | ||
| Increased metabolism – injury, sepsis, malignancy, etc. |
Albumin
Albumin is quantitatively the most important contributor towards maintaining the colloid oncotic pressure of plasma, and hypoalbuminaemia may lead to the development of oedema. Increased albumin concentrations are found in dehydration and if excessive venous stasis is applied during venepuncture. Hypoalbuminaemia may arise due to a number of reasons in addition to the acute-phase response (Table 16.3).
Hypoalbuminaemia
Pathological causes include:
- Reduced synthesis, due to liver disease (p. 199), malnutrition and intestinal malabsorptive disease, if these are severe and prolonged.
- Altered distribution, due to increased capillary permeability, which enables plasma to leak into the extravascular compartment (e.g. severe burns), or to serous effusion (e.g. ascites), when there is sequestration of proteins.
- Increased catabolism, as a result of injury (e.g. major surgery or trauma), infection or malignant disease.
- Abnormal losses, The liver can normally replace up to 5 g/day. Greater albumin losses (which may involve losses of other proteins besides albumin) may occur in nephrotic syndrome, GI tract disease and in burns or certain skin diseases.
- Overhydration, which is usually iatrogenic.
- Artefact due to taking a blood sample from a site close to an IV infusion. This should always be considered (Table 16.3).
Analbuminaemia
This is a rare disorder in which plasma [albumin] is usually less than 1.0 g/L. However, there may be no symptoms or signs – not even oedema – due to compensatory increases in plasma [globulins].
Bisalbuminaemia
Albumin variants occur in the healthy population, and heterozygotes for some variant albumins may express two gene products, which appear as two bands on electrophoresis. This is known as bisalbuminaemia, and has no pathological significance.
C-reactive protein (CRP)
This protein is a β-globulin, originally named after a property of serum that had been obtained from acutely ill patients, which caused the precipitation of a polysaccharide (fraction C) from pneumococcal extracts. CRP binds strongly to certain lipids, particularly phospholipids. It seems that CRP is somehow involved in the body’s response to foreign materials. CRP increases in infection and inflammatory disease processes (up to 20- to 30-fold) and is widely measured as an alternative or adjunct to the less sensitive erythrocyte sedimentation rate (ESR). CRP measurements using a sensitive assay have been used for risk assessment in patients with ischaemic heart disease.
α1-Protease inhibitor (α1-anti-trypsin) (API)
Proteases such as trypsin, chymotrypsin, elastase and thrombin are continually being released into the blood in small amounts from a number of sources, including the pancreas, leucocytes and intestinal bacteria. API is one of several plasma proteins that inhibit the activity of these proteases, particularly neutrophil elastase, and may function to limit proteolytic activity at sites of inflammation. Interest in API principally relates to the association between certain diseases of the lung and liver and API deficiency due to genetic polymorphism.
Clinical consequences of the genetic polymorphism of API
Many allelic genes code for API, the alleles being given the general designation PI (protease inhibitor), and more than 90 genetic variants have been described, some of which result in marked decreases in API activity. The most common allele has been named M, with the usual homozygote being PIMM (the MM type). The two most commont mutations that give rise to API deficiency are the PIZ and PIS alleles. Individuals who are homozygous for the PIZ or PIS alleles are prone to the following diseases.
Pulmonary emphysema
About 1% of patients with emphysema have API deficiency PISS or PIZZ, but this percentage is much higher in young patients. When associated with API deficiency, emphysema tends to manifest itself in the 20–40 year age group. Smoking seems to be a strong predisposing factor for the development of the disease in these patients, possibly because particles in smoke stimulate phagocytic activity, with the local release of proteases.
Hepatic disorders
Neonatal jaundice, usually presenting as a predominantly cholestatic picture, is common in PIZZ individuals. Although the jaundice may resolve, there is usually progression to hepatic cirrhosis. In about 20% of children with cirrhosis, the hepatic disorder can probably be attributed to API deficiency. In adults, cirrhosis and hepatoma are associated with the PiZZ phenotype.
Phenotyping of API by isoelectric focusing is desirable in all cases in which plasma API levels are low or borderline, so that appropriate genetic counselling can be given to affected individuals or to their family members. Genotyping can also be used to investigate relatives of patients with API deficiency and also for antenatal screening.
The immunoglobulins and disease
The Igs are a group of structurally related proteins that function as antibodies; they are synthesised by the plasma cells of the lymphoreticular system.
The basic Ig molecule is made up of four polypeptide chains consisting of a pair of identical heavy chains (Mr 50–75 kDa each) and a pair of identical light chains (Mr 22kDa each) linked by disuphide bridges. There are five principal types of heavy chain (γ, α, μ, δ and ε) and two types of light chain (κ and λ). Every Ig can be assigned a formula that indicates its composition, according to its types of chain (e.g. α2 λ2, γ2 κ2, etc). The antigen-combining sites are between the adjacent light and heavy chains (Figure 16.2).
The immunoglobulin classes
Three major classes of Ig (IgG, IgA and IgM) and two minor ones (IgD and IgE) have been recognised; the type of heavy chain determines the class. Table 16.4 lists several features of the major classes. Both light and heavy chains have ‘constant’ and ‘variable’ sections. The ‘constant’ portion varies little within each particular chain type, whereas the variable portion (which is associated with the antigen-combining site) is different for each Ig, even within a single chain type. The variable portion is responsible for the specificity of the antibody.
Figure 16.2 The Ig molecule, which consists of two identical pairs of heavy and light chains, held together by disulphide bonds (shown as –S–S). The molecules can be split by papain into three components; these are the two antigen-binding fragments (Fab), each of which has one binding site, and the crystallisable fragment (Fc). The variable regions of the Ig molecule are shown as interrupted lines. The heavy chains are one of five types (γ, α, μ, δ or ε), and the light chains are one of two types ( κ or λ).
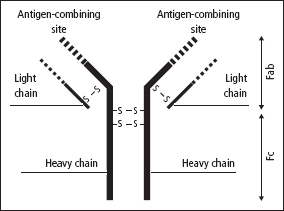
Table 16.4 Some features of the major classes of the Igs

- IgG immunoglobulins comprise the major antibody of the secondary immune response and are formed particularly in response to soluble antigens such as toxins and the products of bacterial lysis. They are widely distributed in the ECF, and cross the foetoplacental barrier.
- IgM immunoglobulins in plasma are pentamers of the basic Ig structure linked around a J chain polypeptide. They tend to be formed especially in response to particulate antigens, such as those on the surface of bacteria. In the presence of complement, IgMs are very effective in producing lysis of these cells. Following an antigenic stimulus, IgM formation usually precedes IgG formation, and IgMs are thought to provide an early defence mechanism against intravascular spread of infecting organisms.
- IgA immunoglobulins as they occur in plasma, are monomers. However, over 50% of IgA synthesis occurs in lymphoreticular cells under the mucosa of the respiratory and alimentary tracts. Here dimeric ‘secretory IgA’ is synthesised and secreted into the alimentary or respiratory tract and may form part of the defence mechanism against local infections.
- IgD immunoglobulins are present in minute amounts in plasma. They are also often present, with monomer IgM, on the surface of B lymphocytes. They are probably concerned with antigen recognition and with the development of tolerance.
- IgE immunoglobulins bind to cells such as the mast cells of the nasopharynx. In the presence of antigen (allergen), an antigen–antibody reaction leads to the release of histamine and other amines and polypeptides from the mast cell, giving rise to a local hypersensitivity reaction.
Immunoglobulin deficiencies
Identification of Ig deficiencies requires quantification of specific classes of Ig. Low concentrations of Igs may be due to a variety of causes.
Physiological causes
The concentrations of IgM and IgA in serum are low at birth and gradually rise until adult levels are achieved at approximately 1 year and 10 years, respectively. IgG is high at birth due to transplacental passage of maternal IgG. After birth IgG falls due to loss of maternal IgG but then gradually rises again until adult values are found after 1 year.
Inherited deficiencies of immunoglobulin synthesis
Hypogammaglobulinaemia and, rarely, agammaglobulinaemia are conditions in which there is defective production of IgG, IgA and IgM. Children develop severe, recurrent bacterial infections when over the age of 1. The most common is IgA deficiency which has an incidence of approximately 1 : 400.
Acquired deficiencies of immunoglobulin synthesis
Secondary hypogammaglobulinaemia is much more common than the inherited deficiencies. It may occur in lymphoid neoplasia (e.g. chronic lymphatic leukaemia, Hodgkin disease, multiple myelomatosis), in ‘toxic’ disorders or certain types of drug therapy, in protein-losing syndromes (e.g. nephrotic syndrome), and in prematurity and delayed maturity.
Hypergammaglobulinaemia (polyclonal – diffuse)
Liver disease, infection and autoimmune disease gives rise to stimulation of B lymphocytes and an increased production of γ-globulin, which on serum protein electrophoresis is revealed as a broad (diffuse) band (Figure 16.1). The increase may affect all the Ig classes, or it may affect predominantly one class. The antibodies produced are heterogeneous. Quantitation of the separate Ig classes is occasionally helpful in diagnosis. In most cases, however, the cause of the diffuse increase in plasma [total Ig] is apparent. The measurement of antibodies to specific antigens is of value (e.g. hepatitis surface antigens). Multiple discrete bands (oligoclonal bands) or, rarely, a single discrete band may occur in the λ-globulin region in response to an antigenic stimulus.
Hypergammaglobulinaemia – (monoclonal – discrete – paraproteinaemia)
A paraprotein is a monoclonal Ig or light chain produced by a clonal population of B cells. They are often identified as a discrete Ig band on electrophoresis of serum (figure 16.1). Plasma cell disorders are often associated with multiple myeloma and malignant lymphoid tumours, but benign causes are also described. The detection of a paraprotein in blood or urine requires further investigation to determine whether the paraproteinaemia is malignant or benign (see below).
Myeloma, lymphoid malignancies and paraproteinaeimea
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


