Fig. 5.1.
Patients awaiting organs in the United States, from United Network for Organ Sharing (UNOS, http://optn.transplant.hrsa.gov/).
General Considerations
Transplant Recipient Candidate
♦
Waiting list for most organs is long and many patients die awaiting a suitable organ
♦
Good health except for the failed organ
♦
Rarely multiple organs can be transplanted together
Heart and lung for congenital heart disease
Pancreas and kidney together for type 1 diabetes
Small intestine and other abdominal organs for functional short gut and subsequent total parenteral nutrition (TPN)-induced liver injury
♦
Age is not a definite excluding factor in the USA
However, it may be technically impossible to perform the surgery on small premature infants
Older patients (>70 years old) often have multiple diseased organs that may exclude them from consideration
♦
Cardiac, pulmonary, liver, and renal function are assessed for adequacy
Significant multi-organ disease may disqualify someone from transplantation
♦
High-grade malignancy, e.g., metastatic colon cancer, a general contraindication to transplantation because of cancer recurrence and death
♦
HIV infection no longer a barrier to transplantation
♦
Significant previous surgery at the allograft site may have induced adhesions or altered anatomy such that a transplant cannot be performed
♦
Primary amyloidosis is a general contraindication to transplant as it quickly recurs in the allograft
Some exceptions for hereditary amyloid where liver/cardiac transplant can be life prolonging
♦
Must be psychosocially appropriate – patient must be able to assist in one’s own care, take medications, return for follow-up visits, etc
Family support is crucial to the success of the transplant
Social workers and psychologists are integral members of the transplant team
♦
Suitable transplant candidates are placed on waiting list
♦
Factors affecting length of time on the waiting list may include availability of organ, tissue match, blood type, immune status, disease acuity, and the distance between the potential recipient and the donor
♦
Heart, liver, and intestine patients – sickest transplanted first
Organ Donor
♦
Donor suitability – healthy (no viral infections, no rapid onset dementia, no high-grade malignancy), “normal” organs
♦
Donations facilitated by United Network for Organ Sharing (UNOS, http://www.unos.org – transplant survival data by organ and transplantation center), which divides the USA into 11 regions
The goal is to reduce organ preservation time, improve organ quality and survival outcomes, reduce costs incurred, and provide governance of the process at a local level
♦
Harvested organs distributed in region first
♦
If no suitable recipient in region, offered nationwide
♦
In general, organs are harvested from brain-dead donors and perfused with Wisconsin solution (proprietary) to prolong extracorporeal usefulness
♦
Heart and lung have short “shelf life” of 8–10h; liver and kidney up to 24h
♦
Organ and donor are assessed for blood group system (ABO) and HLA and screened for antibody incompatibility and lymphocyte cross match (serum from the recipient)
Depending on the organ and the clinical situation, some or all of these factors may not enter into a decision to use the organ in a particular patient
Other criteria may also be important, i.e., size and weight depending on the organ
Simplified Transplantation Immunology
♦
Allograft rejection is a complex, not completely understood process, involving both the T- and B-cell arms of the immune system that results in damage and functional impairment to the allograft organ (Fig. 5.2)
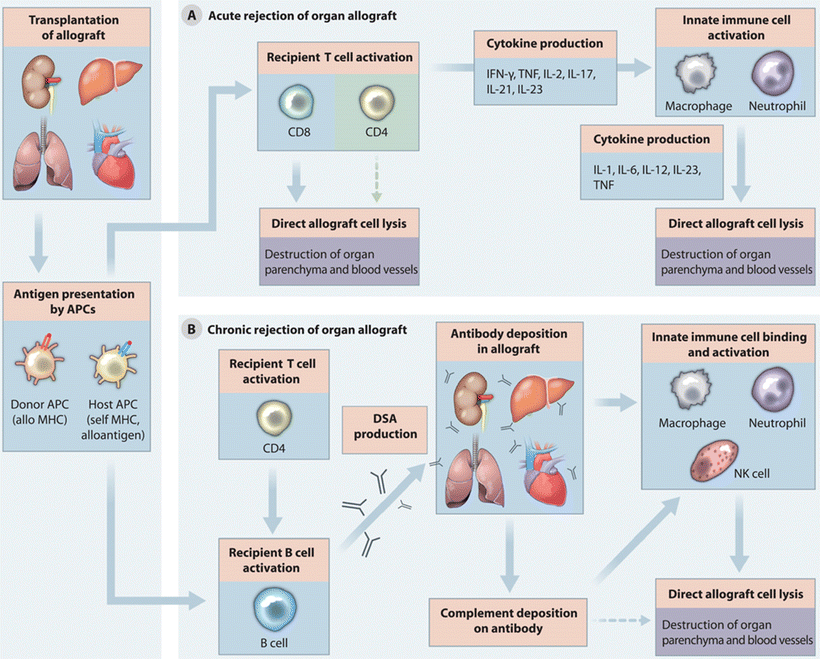

Fig. 5.2.
Summary of the rejection process. Acute cellular rejection (A) is mediated initially by antigen-presenting cells (APC) from the donor. They present antigen to recipient T cells resulting in T-cell activation, proliferation, and cytokine production. Cytokines recruit additional inflammatory cells leading to graft injury. As the donor APC cells become deleted over time, host APC cells fill that role leading to possible immune tolerance. In chronic rejection (B) APCs contribute to B-cell proliferation, activation, and maturation into plasma cells. Donor-specific antibodies (DSAs) bind to graft endothelium triggering the complement cascade which results in graft injury. Reprinted with permission from McDonald-Hyman C et al. Advances and challenges in immunotherapy for solid organ and hematopoietic stem cell transplantation. Sci Transl Med. 2015;25:280rv2.
Cellular or T-Cell-Mediated Rejection
♦
The major histocompatibility complex (MHC) of the immune system that governs “self vs. other” resides in three loci on chromosome 6 – the human leukocyte antigens (HLAs)
♦
These HLA loci are inherited in a Mendelian-dominant fashion; each person with two haplotypes
♦
Class I MHC antigens are present on the surface of all nucleated cells
♦
The class I antigen is composed of three alpha chains (coded by genes HLA-A, HLA-B, and HLA-C) noncovalently bound to beta2-microglobulin
♦
Class II antigens are found on activated T cells, monocytes, macrophages, Langerhans cells, dendritic cells, endothelium – antigen-presenting cells (APCs) and certain other cells
♦
Class II antigens are a heterodimer composed of an alpha and a beta chain coded by the HLA-D region
♦
Both class I and class II molecules contain peptide-binding sites
♦
The allograft activates recipient T cells via direct and indirect allorecognition
♦
Direct allorecognition – donor APC cells migrate to recipient lymphoid organs and interact with recipient T cells
Principle mechanism of acute cellular rejection
♦
Indirect allorecognition – recipient APC cells bind donor antigen and interact with the T cells
As donor APC become deleted over time, indirect allorecognition becomes the driving force of cell-mediated rejection
Natural killer (NK) cells also play a role in the process of allograft destruction but work through a somewhat different pathway.
♦
May play a role in graft tolerance.
♦
T-cell activation is multifactorial
APC cell binds to the T-cell receptor on the T cell
“Costimulation” by other cell surface antigens (CD28, CD40, CD40L, B71, B72) required for activation
Lack of costimulation results in anergy
The above causes increased intracellular calcium via tyrosine kinase pathways in the T-cell activating calcineurin
Activated calcineurin dephosphorylates nuclear factor for activated T cells (NFATs), which is then transported into the nucleus
NFAT binds to the IL-2 promoter, increasing the synthesis of IL-2
Increased IL-2 causes increased DNA synthesis by binding to IL-2 receptors and recruits additional T cells
♦
NK cells and activated T cells destroy the allograft (cellular rejection)
Immunosuppression
♦
Many immunosuppressive agents appear to interfere with T-cell activation or proliferation, thereby inducing temporary tolerance of the graft
♦
Common agents include:
OKT3, antilymphocyte globulin (ALG), and antithymocyte globulin (ATG)
Interferes with antigen presentation
♦
Can induce serum sickness; induce antibodies
Corticosteroids
Block T-cell proliferation
♦
Numerous side effects (glucose intolerance, osteoporosis, etc.)
Calcineurin inhibitors: cyclosporin A, tacrolimus (FK506)
Powerful immunosuppressants
♦
Nephrotoxic
Azathioprine
Blocks cell replication
♦
Causes bone marrow suppression
Mycophenolate mofetil
Blocks cell replication.
Causes bone marrow suppression.
Can produce gastrointestinal (GI) symptoms including diarrhea.
♦
Colonic biopsies of mycophenolate toxicity may show inflammatory bowel disease-like inflammation or crypt apoptosis (graft vs. host disease [GVHD]-like changes).
Rapamycin (sirolimus)
Blocks IL-2-driven cell cycle progression
♦
May induce bone marrow suppression
Basiliximab, daclizumab, etc
♦
Anti-IL-2 receptor antibodies
Alemtuzumab (CamPath)
Binds to CD52 on both T cells and B cells leading to marked depletion of these cell types
♦
A host of new immunosuppressive agents are being developed
Promise more efficacy and less toxicity (Fig. 5.3)
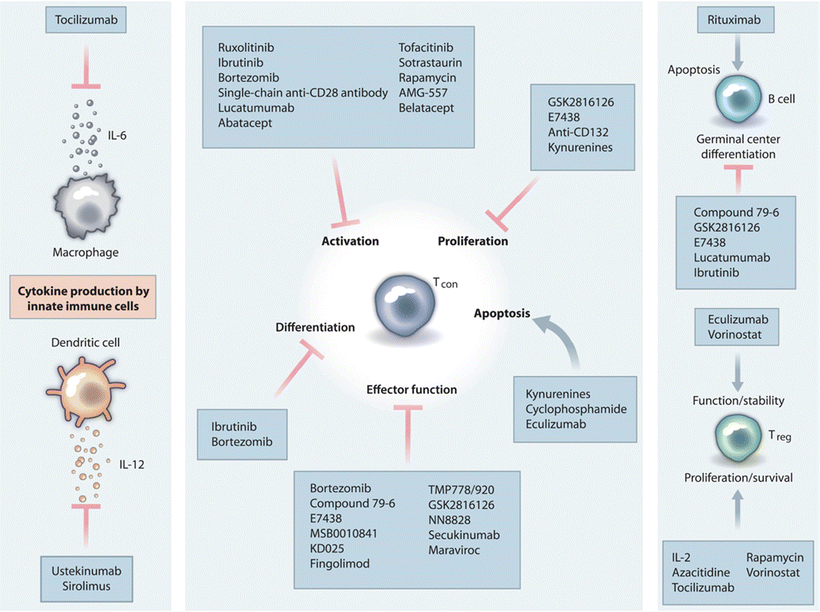
Fig. 5.3.
Antirejection medications interfere with various parts of the immune process including T- and B-cell activation, differentiation, proliferation, apoptosis, and effector function. Other medications interfere with cytokines or their production. Some of the targets of newer antirejection medication are illustrated here. Reprinted with permission from McDonald-Hyman C et al. Advances and challenges in immunotherapy for solid organ and hematopoietic stem cell transplantation. Sci Transl Med. 2015;25:280rv2.
♦
A combination of immunosuppressive agents is usually employed, idiosyncratic to the institution and the organ
♦
Immunosuppression is a careful balancing act – too little immunosuppression leads to graft destruction; too much immunosuppression leads to uncontrollable opportunistic infection, viral reactivation [cytomegalovirus (CMV), human papillomavirus (HPV), Epstein–Barr virus (EBV), hepatitis B virus (HBV), hepatitis C virus (HCV), etc.] in infected individuals, and posttransplant lymphoproliferative disorder (PTLD)
Humoral or Antibody (B-Cell)-Mediated Rejection
♦
Contact with alloantigens (transfusion, pregnancy, transplantation) may produce alloantibodies leading to humoral rejection
♦
Donor-specific antibodies (DSAs) – antibodies directed against class I and II HLA molecules
Patients with preformed or acquired DSA experience greater than average graft loss
Other non-HLA-related antibodies to unmasked, usually hidden epitopes may developed over time and contribute to chronic graft failure, i.e., vimentin or type V collagen
♦
Hyperacute rejection
In the case of strong preformed antibodies, such as an ABO incompatibility, there is almost immediate graft failure
Heart and kidney most likely to be affected
In this situation, the antigen–antibody complex deposits diffusely in the microvasculature triggering complement activation leading to thrombosis, recruitment of neutrophils, congestion, edema, and graft dysfunction
♦
Acute (subacute) rejection
However, weaker antigen–antibody interactions can occur days to several months later
The process is similar but tends to be patchier
Small arterioles are also affected
♦
Chronic (late) rejection
Most of the alloantibodies are directed against HLA-related antigens
However, recently, there has been evidence to suggest that some chronic (late) humoral rejection may be related to non-HLA antigens such as vimentin and type V collagen
The main endothelial damage tends to affect moderate-sized arteries and arterioles
As these vessels become occluded (so-called transplant vasculopathy), the graft becomes ischemic and dysfunctional
♦
Nonhyperacute rejection
Detection of nonhyperacute humoral rejection can be difficult
The histologic changes are often subtle with slight neutrophil margination and endothelial swelling
Damage to larger vessels may not be detectable in small samples such as biopsies
Circulating antibody–antigen complexes can be detected by a number of methods including lymphocytotoxicity assays, flow cytometry, ELISA, and a Luminex solid-phase assay
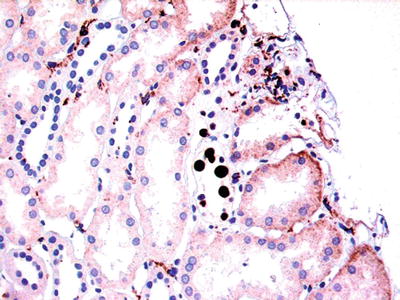
After the antibody–antigen complex binds to the endothelium, the complement cascade is triggered (Fig. 5.4)
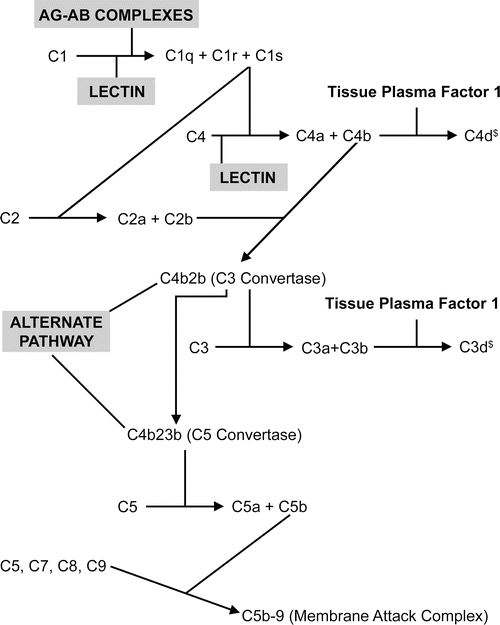
Fig. 5.4.
Schematic of compliment activation after antibody binds to endothelium in humoral rejection. The dark lines indicate entities that mediate the reaction. § indicates tissue bond entity. Reprinted with permission from Truong LD, Barrios R, Adrogue HE, Gaber LW. Acute antibody mediated rejection of renal transplant: pathogenetic and diagnostic considerations. Arch Pathol Lab Med. 2007;131:1200–8.
Two complement breakdown products, C4d and C3d, become covalently attached to the injury site
Immunofluorescence studies on fresh tissue can identify immunoglobulin and complement in the vessels as a marker of humoral rejection
C4d can be detected by immunoperoxidase methods using formalin-fixed paraffin-embedded tissue as well as fresh tissue.
It has been touted as a good marker of humoral rejection in the kidney and heart, in general practice.
♦
Therapy for humoral rejection is evolving but suboptimal
Usually the dosages of standard immunosuppressive drugs are increased and the patient undergoes plasmapheresis
The addition of rituximab to this regimen may be beneficial but further investigations are ongoing
♦
There is hope that after an unknown period of time, immunosuppression may no longer be needed because of graft tolerance or chimerism
Complications Common to All Transplants
Infection
Nonopportunistic
♦
Identical in clinical and histologic findings to those seen in the nontransplant patient; common sites and agents include:
Pneumonia: Pseudomonas, Staphylococcus, Streptococcus, Enterobacteriaceae
Sepsis: Staphylococcus, Enterobacteriaceae, Pseudomonas, Enterococcus, anaerobes
Wound infection: Staphylococcus, Pseudomonas, Enterococcus, Enterobacteriaceae, anaerobes, mixed flora
Urinary tract: Enterococcus, Enterobacteriaceae, Pseudomonas
Opportunistic
♦
Virus
♦
Cytomegalovirus
Most common infection after transplantation
~80% adult population exposed to and harbor latent virus
♦
All transplant patients are given prophylactic antiviral therapy.
Typically noted 30+ days after the transplant
Classic nuclear enlargement with intranuclear and cytoplasmic inclusions (Fig. 5.5)
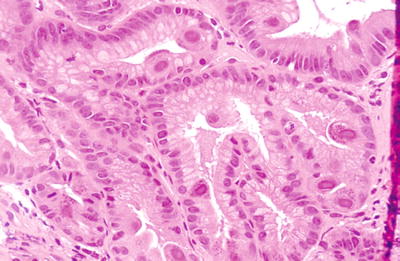
Fig. 5.5.
Cell with nuclear enlargement and classic CMV nuclear and cytoplasmic inclusions in gastric mucosa. Inflammatory reaction to the virus is variable with little inflammation seen here. Levels into the block are helpful. If only the nuclear inclusions are noted, it might be confused for herpes- or adenovirus. If only the cytoplasmic inclusions are found, they may be confused with toxoplasma.
Present with graft dysfunction, possibly fever
Culture, immunohistochemistry, or molecular methods may aid diagnosis
Occurs in the graft itself or nontransplanted organs
Treated with ganciclovir or other antiviral agents and lowered immunosuppression
♦
Adenovirus
Most common in children but can be seen in adults
Classic intranuclear inclusions
Usually seen in the gut, liver, or lung
♦
Herpesviruses
Classic intranuclear inclusions (Fig. 5.6A, B)
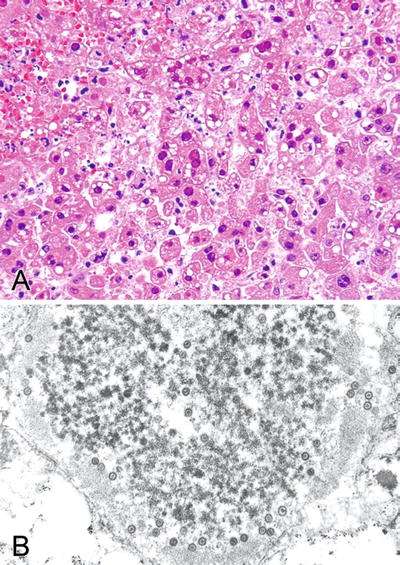
Fig. 5.6.
(A) Liver with classic herpes hepatitis. Only ground-glass nuclear inclusions with little or no nuclear enlargement are seen with this virus. (B) Ultrastructural examination of the liver shows classic targetoid particles of herpesvirus. This technique is useful is distinguishing herpes inclusions from adenovirus inclusions.
Brain, skin, and liver are most commonly affected
♦
Epstein–Barr virus
Common virus, >50% of the adult population exposed and harbor latent virus
EBV-negative donor organ matched to negative recipient to prevent posttransplant lymphoproliferative disease
In situ hybridization (EBER) employed for detection
♦
Protozoan
Pneumocystis
Classic pulmonary histology (Fig. 5.7A, B) treated in the usual fashion
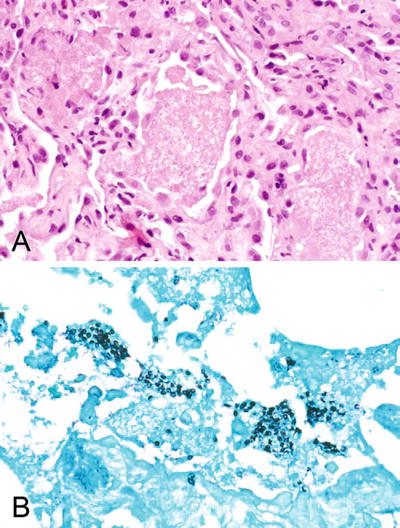
Fig. 5.7.
(A) Lung with classic foamy alveolar exudates and slight chronic inflammation of the interstitium consistent with Pneumocystis. (B) Gomori methenamine silver (GMS) stain of (A) showing classic Pneumocystis cysts.
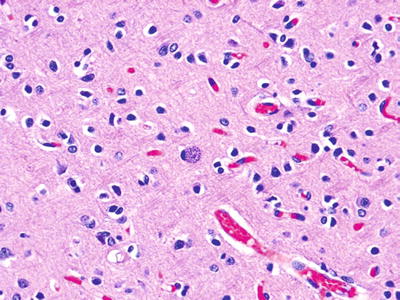
Fig. 5.8.
Toxoplasma cyst from the brain from a 7-year-old child status post-bone marrow transplant for acute lymphocytic leukemia (ALL).
Most transplant patients are given prophylactic treatment to prevent infection.
Toxoplasmosis
Inflammatory infiltrates may be seen in the heart, lung, and brain associated with classic cytoplasmic inclusions (Fig. 5.8).
♦
Fungi
Candida
Can involve any site
Classic pseudohyphae well visualized with silver stain (GMS) or PAS
Aspergillus
Angioinvasive hyphae often present with infarction of involved organ.
Neoplasia
Condyloma
♦
Immunosuppression causes increased viral proliferation
♦
Condylomas very common – HPV
De Novo Carcinogenesis
♦
De novo carcinogenesis is also common
♦
Cutaneous squamous cell carcinoma most common
♦
Kaposi sarcoma
Posttransplant Lymphoproliferative Disease
♦
PTLD is a lymphoid and plasma cell proliferation that develops in immunosuppressed patients most commonly secondary to EBV infection
♦
The disease incidence is difficult to quantitate because of variability due to organ type, patient age, and immunosuppression regimen
♦
The general incidence is between 1% and 5%, more common in children and patients on high immunosuppression regimens such as small bowel allografts
♦
The most important predisposing factor is seronegativity for EBV at the time of transplantation
♦
The majority of PTLD in solid organ transplant patients is donor derived; however, in bone marrow transplant (BMT) patients, the reverse is true
♦
The allograft itself may be involved although this is uncommon in heart transplant patients
♦
Nonallograft organ involvement may be seen including the lung, liver, GI tract, spleen, lymph nodes, and central nervous system
It may be multifocal
Most commonly in BMT patients
♦
PTLD may be divided into three major types: early, polymorphous, and monoclonal
Early (Plasma Cell Hyperplasia and Mononucleosis-Like)
♦
Early type is seen largely in children and seronegative adults representing their first EBV infection
♦
It presents with fever, pharyngitis, and lymphadenopathy generally reflecting the location of the lesion
♦
The tonsils, adenoids, and nodes are enlarged but the underlying architecture is preserved
♦
Histology exhibits numerous plasma cells, lymphocytes, and occasional immunoblasts or paracortical expansion with numerous immunoblasts in a background of T cells and plasma cells
♦
Usually EBV and a polyclonal B-cell immunophenotype can be demonstrated
♦
The lesion responds favorably to decreased immunosuppression
Polymorphous
♦
Polymorphous variant is usually seen within the first year after solid organ transplantation, 6 months for BMT
♦
Presentation is variable, sometimes with mass effect and/or graft dysfunction
Systemic symptoms may or may not be present
♦
It may occur in the graft itself or in nongrafted sites; bone marrow involvement is rare
♦
Polymorphous PTLD histology demonstrates a dense polymorphous mononuclear infiltrate with prominent immunoblasts often exhibiting easily found mitotic figures and plasma cells effacing the underlying architecture (Fig. 5.9)
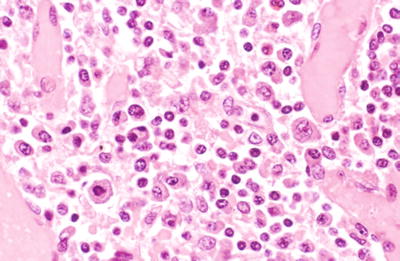

Fig. 5.9.
Enlarged lymph node showing a pleomorphic infiltrate including numerous plasma cells and large plasmacytoid cells with scattered immunoblasts consistent with polymorphous PTLD. Immunostaining for EBV was positive (not shown).
♦
The infiltrate should not be characteristic of a classic lymphoma type
♦
EBV can be demonstrated by culture, immunohistochemistry, or molecular techniques in most cases (>70%)
♦
Flow cytometry, genomic analysis, or immunohistochemistry shows a predominantly polyclonal B-cell proliferation
♦
The large atypical cells may be CD30 positive but lack CD15
♦
Late lesions (4+ years after transplantation) are more likely to be EBV negative
♦
HHV-8 has been identified in some of these EBV-negative cases
♦
Polymorphous PTLD often responds to lowered immunosuppression; however, the mortality may still run 20–40%
Monoclonal
♦
Monoclonal variant of PTLD usually presents as a mass involving the graft itself or in a nongrafted site
♦
Histology of the lesion shows a dense monotonous mononuclear infiltrate that effaces the architecture reminiscent of typical B-cell lymphomas (Fig. 5.10)
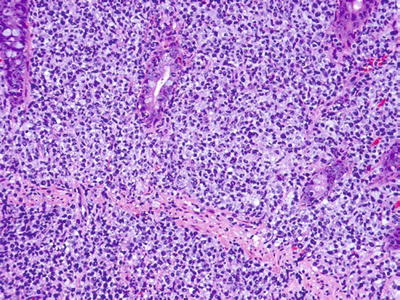

Fig. 5.10.
Colon mass showing a diffuse, relatively monotonous infiltrate of large atypical lymphocytes of PTLD lymphoma. Immunostaining for EBV was negative (not shown).
♦
The B-cell tumors are usually diffuse high-grade lesions but T-cell lymphomas can also be seen
♦
EBV is less easily demonstrated than in the abovementioned types
♦
Flow cytometry and/or immunohistochemical studies typically show a monoclonal B-cell proliferation, but gene rearrangement studies may be needed if it is a T-cell lesion
♦
Treatment consists of lowered immunosuppression and lymphoma chemotherapy, but the mortality can be as high as 70%
Variants
♦
Some authors include a rarely seen Hodgkin-like variant
♦
Non-EBV-driven lymphomas can also develop
They have the typical presentation and histology of lymphoma occurring outside the transplant milieu
Lymphomas derived from mucosa-associated lymphoid tissue (MALT) should be placed in this category
Outcome depends on the lymphoma type
Rejection
♦
General patterns
Hyperacute Rejection
♦
Immediate graft failure secondary to preformed antibodies
♦
Marked congestion – may see fibrin platelet thrombi in capillaries
Acute Cellular Rejection
♦
Seen first 7–10 days after transplant
♦
Organ dysfunction often clinically evident
♦
Mixed mononuclear infiltrate of lymphocytes, lymphoblasts, and eosinophils attacking epithelial structures
♦
Endotheliitis – lymphocytes associated with and disrupting endothelium (usually with enlarged chromatic nuclei) of small veins may be seen
♦
Usually responds to increased immunosuppression
♦
Precursor to chronic rejection
Chronic Cellular Rejection
♦
Later occurrence but can be seen as early as 2 months posttransplant but usually one or more years later
♦
Severe organ dysfunction
♦
Mononuclear infiltrate associated with marked destruction, loss, or fibrosis of epithelial structures
♦
Often associated with transplant arteriopathy
♦
Not easily treated, often results in graft failure
Acute Vascular Rejection
♦
Necrotizing or cellular vasculitis
♦
Usually associated with severe cellular rejection
♦
Responds poorly to therapy
Chronic Vascular Rejection (Transplant Vasculopathy)
♦
Diffusely involves moderate-sized arterioles
♦
Foamy intimal change
♦
Concentric fibromuscular proliferation with an intact internal elastica
♦
Usually associated with chronic cellular rejection
♦
Not easily treated, often seen in failed grafts
Disease Recurrence
♦
Organ dependent – see below
Drug Toxicity
♦
Probable common cause of morbidity in the transplant patient
♦
Poorly recognized, a diagnosis of exclusion
♦
Osteoporosis, hypertension, and glucose intolerance (type 2 diabetes) are common side effects of immunosuppression
Kidney Transplantation
Indication for Transplantation
♦
Indication for transplantation is generally chronic renal failure produced by sundry injuries including hypertension, diabetes, glomerulonephritis (GN), etc
Clinical Considerations
♦
Cadaveric and living related donors used
Living related organs fair slightly better (95% 1-year graft survival compared with 88% graft survival for cadaveric grafts)
♦
Allograft usually placed ectopically in the pelvic fossa
♦
Renal artery and vein anastomosed to iliac vessels; ureter tunneled into urinary bladder
♦
Native kidneys are usually left in situ except in adult polycystic kidney disease
Infection and neoplasia risk
May develop renal cell carcinoma
♦
Donor organ sometimes biopsied for evaluation of arteriolonephrosclerosis (Fig. 5.11)
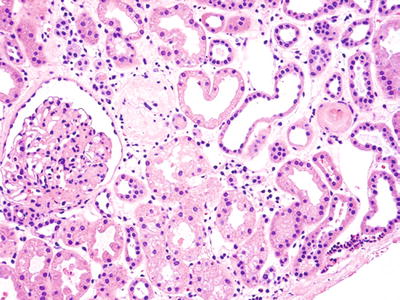

Fig. 5.11.
Wedge biopsy of kidney prior to transplantation to evaluate extent of underlying disease. Capsule is seen in lower right corner. There is patchy interstitial fibrosis, intact and occasional senescent glomeruli, and hyaline arteriolosclerosis. The organ was used successfully for transplantation.
♦
We report the number of sclerotic glomeruli vs. the total number of glomeruli
Rejection
Kidney Biopsy for Rejection
♦
Presents with elevation of creatinine 7–10 days or longer after transplantation
♦
Diagnoses by cutting needle biopsy obtained often with ultrasound guidance
♦
Formalin fixation generally adequate but frozen (for immunofluorescence) and glutaraldehyde-fixed specimens (for ultrastructure) may be obtained if disease other than rejection is suspected
♦
Five or more glomeruli considered adequate for diagnosis
♦
At least two H&Es plus connective tissue stains and PAS are typically evaluated
Banff Criteria
♦
Banff consensus scheme for evaluating renal transplant biopsies has evolved over time, the latest iteration provided in Table 5.1
Table 5.1.
BANFF 2007 Consensus Classification of Renal Allograft Pathology
1. Normal |
2. Antibody–mediated changes See Table 5.2 |
3. Borderline changes: “Suspicious” for acute T-cell-mediated rejection (may coincide with categories 2, 5, and 6). This category is used when no intimal arteritis is present, but there are foci of tubulitis (t1, t2, or t3) with minor interstitial infiltration (i0 or i1) or interstitial infiltration (i2, i3) with mild (t1) tubulitis |
4. T–cell–mediated rejection (TCMR) (may coincide with categories 2, 5, and 6) Acute T-cell-mediated rejection (type/grade): IA. Cases with significant interstitial infiltration (>25% of parenchyma affected, i2 or i3) and foci of moderate tubulitis (t2) IB. Cases with significant interstitial infiltration (>25% of parenchyma affected, i2 or i3) and foci of severe tubulitis (t3) IIA. Cases with mild-to-moderate intimal arteritis (v1) IIB. Cases with severe intimal arteritis comprising >25% of the luminal area (v2) III. Cases with “transmural” arteritis and/or arterial fibrinoid change and necrosis of medial smooth muscle cells with accompanying lymphocytic inflammation (v3) Chronic active T-cell-mediated rejection “Chronic allograft arteriopathy” (arterial intimal fibrosis with mononuclear cell infiltration in fibrosis, formation of neointima) |
5. Interstitial fibrosis and tubular atrophy, no evidence of any specific etiology (may include nonspecific vascular and glomerular sclerosis, but severity graded by tubulointerstitial features) Grade I. Mild interstitial fibrosis and tubular atrophy (<25% of cortical area) II. Moderate interstitial fibrosis and tubular atrophy (26–50% of cortical area) III. Severe interstitial fibrosis and tubular atrophy/loss (>50% of cortical area) |
6. Other. Changes not considered to be due to rejection – acute and/or chronic; may include isolated g, cg, or cv lesions and coincide with categories 2–5 |
It is a complex system mainly divided into humoral rejection, cellular rejection, and other based on evaluation of a number of subparameters.
♦
Category 2: antibody-mediated changes
“C4d deposition without morphologic evidence of active rejection” is a new category of antibody-mediated rejection (AMR)
In this category, the patient has circulating antibodies and C4d deposition in the graft but no other signs of humoral (AMR) or cellular (TCMR) allograft rejection or injury
Some examples of injury are given including no glomerulitis (g0), no double contour glomerular basement membranes (cg0), and no peritubular capillary inflammation (ptc0) or laminations
C4d deposition is considered to be present when more than 50% of the peritubular capillaries show positive staining via the immunoperoxidase method
Arterial and venous staining is not considered
The clinical significance of this category is unclear, but its presence should be an indication for careful clinical follow-up
♦
AMR
Clinically, this may be correlated with two syndromes
In hyperacute (immediate) rejection when the graft is revascularized on the operating room table, it becomes dusky and fails to make urine.
There is no useful therapy available, and it results in immediate graft loss because of preformed antibodies.
It is an uncommon problem in the modern era, but when seen it may have been due to inadvertent ABO incompatibility.
There are now protocols to pretreat patients such that ABO-incompatible grafts survive almost as well as matched grafts.
Delayed hyperacute (accelerated acute) rejection can also be seen.
It occurs several hours to several days after the transplant and presents with increased creatinine and decreased urine flow; it may be reversible with appropriate treatment (Table 5.2).
Table 5.2.
Antibody-Mediated Rejection in the Kidney
Acute / Active Antibody Mediated Rejection in the Kidney (all 3 required for diagnosis)
1. Histologic evidence of acute tissue injury including one or more of the following:
(a) Microvascular inflammation
(b) Intimal or transmural arteritis
(c) Acute thrombotic microangiopathy (no other cause apparent)
(d) Acute tubular injury (no other cause apparent)
2. Evidence of antibody interaction with vascular endothelium
(a) Linear C4d staining in peritubular capillaries
(b) At least moderate microvascular inflammation
(c) Gene transcripts from the biopsy indicative of endothelial injury
3. Serologic evidence of donor-specific antibodies (DSA)
Chronic active antibody–mediated rejection in the kidney (all 3 required for diagnosis)
1. Morphologic evidence of chronic tissue injury
Plus 2 and 3 as above
♦
Chronic active AMR
Chronic active AMR presents with progressive loss of graft function (rising creatinine) over months to years, often with proteinuria and hypertension (Table 5.2)
♦
Categories 3 and 4, T-cell-mediated rejection (TCMR )
The borderline category is relatively self-evident but is based on evaluating the extent of tubulitis (Table 5.3) and interstitial inflammation
Table 5.3.
Grading of Tubulitis (Applies to Nonatrophic Tubules)
t0
No mononuclear cells in the tubules
t1
Foci with 1–4 cells/tubular cross section (or 10 tubular cells)
t2
Foci with 5–10 cells/tubular cross section
t3
>10 cells/tubular cross section or the presence of at least two areas of basement membrane destruction accompanied by significant interstitial inflammation and t2 tubulitis elsewhere in the biopsy
Significant interstitial inflammation is defined as >25% of the parenchyma being inflamed.
Borderline cellular rejection is tubulitis with insignificant interstitial inflammation or t1 tubulitis with significant interstitial inflammation.
Acute TCMR is divided into five subgrades
Grade I rejection shows significant interstitial inflammation with t2 tubulitis (grade IA) or t3 tubulitis (grade IB).
If arteritis is present, grades IIA, IIB, and III are reported depending on the grade of the vasculitis (Table 5.4).
Table 5.4.
Vasculitis Grading Scheme
v0
No vasculitis
v1
Mild-to-moderate intimal arteritis in at least one arterial cross section
v2
Severe intimal arteritis with at least 25% of cross-sectional area lost in at least one arterial cross section
v3
Transmural arteritis and/or arterial fibrinoid change and medial smooth muscle necrosis with lymphocytic infiltrate in the vessel
Chronic active TCMR refers to the chronic vasculopathy that is common to all transplant sites
It may have a significant humoral component as well.
♦
Category 5, interstitial fibrosis and tubular atrophy, and category 6, other acute or chronic injuries, refer to fibrosis and/or degenerative changes that do not appear to be due to rejection but may be related to hypertension, diabetes, or other disorders
The term chronic transplant nephropathy/glomerulopathy has been supplanted by more specific diagnoses such as chronic AMR or others as the histology dictates
♦
The rejection grades themselves are somewhat difficult to illustrate in a limited space because of the many permutations of the main categories 2–5
Some features of classic cellular rejection are shown in Fig. 5.12A–F
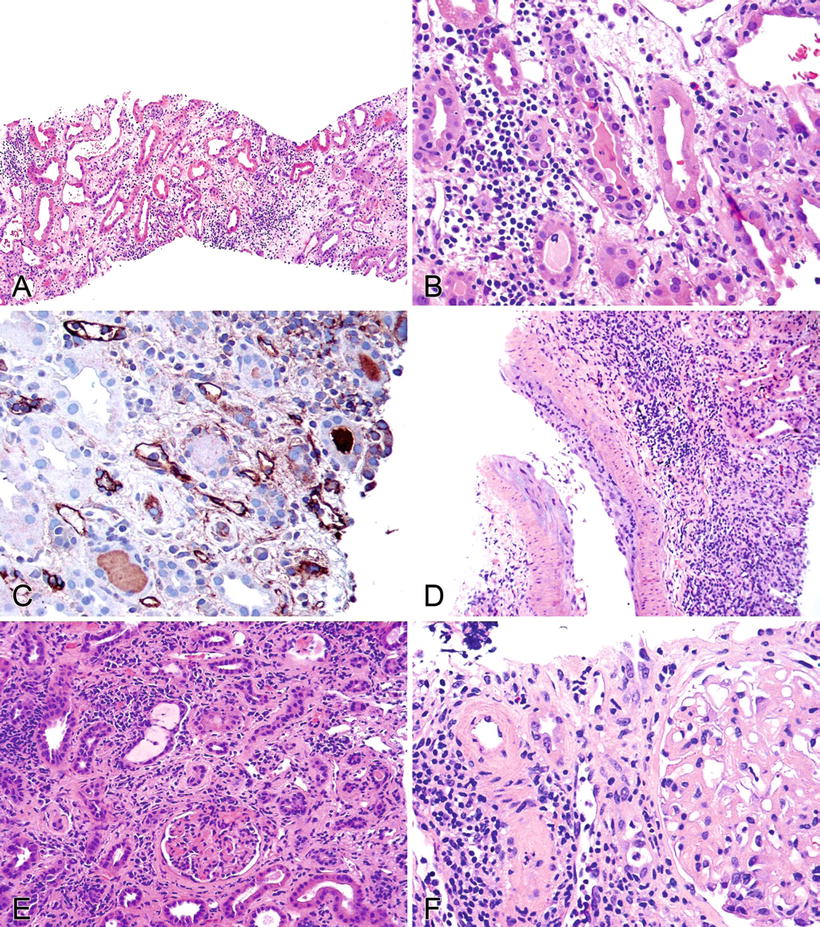
Fig. 5.12.
(A) Allograft kidney showing an interstitial edema and a mononuclear cell infiltrate occupying >25% of the parenchyma, a significant interstitial infiltrate. (B) Allograft kidney showing 5–10 mononuclear inflammatory cells infiltrating the tubular epithelium – t2 tubulitis. (C) Immunoperoxidase stain for C4d showing positive staining in peritubular capillaries consistent with antibody-mediated rejection. (D) An arteriole with endothelial and mural inflammation, a v1 vasculitis. (E) Kidney allograft with chronic interstitial inflammation and fibrosis. The glomeruli also appear to show loop abnormalities. Without additional information, one cannot distinguish chronic rejection from recurrent or de novo glomerulonephritis. (F) Arteriosclerosis is seen here with a background of interstitial inflammation and fibrosis. This was a hypertension-related lesion.
Figure 5.12A illustrates significant interstitial inflammation
If coupled with t2 tubulitis (Fig. 5.12B), the patient would have T-cell-mediated acute cellular rejection IA
If C4d were also present (Fig. 5.12C), it would be both cellular and humoral rejection
If the interstitial inflammation and tubulitis were coupled with v1 arteritis (Fig. 5.12D), the patient would have grade IIA cellular rejection
Interstitial fibrosis and tubular atrophy can be seen in Fig. 5.12E
♦
Other nonrejection-related pathology
Nonrejection-mediated medial arterial changes can be seen in Fig. 5.12F
The glomerulus is abnormal and needs to be evaluated further with specials stains, immunofluorescence, or ultrastructural examination
Fig. 5.13A, B shows glomerular double-contoured loops that must be interpreted in context of the rest of the biopsy
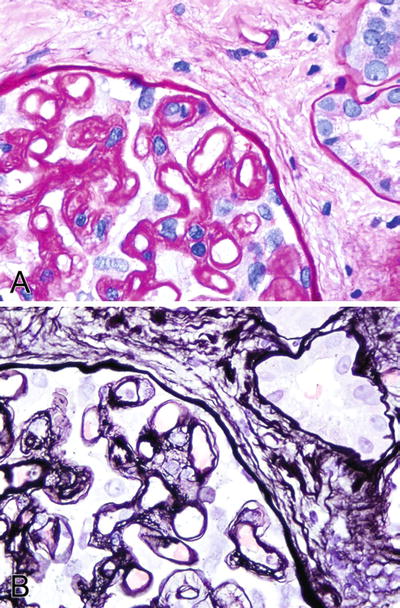
Fig. 5.13.
(A) PAS stain highlighting the double contour glomerular capillary loops. (B) Silver stain (Jones) showing similar findings.
Recurrent Disease
Glomerulonephritis
♦
Recurs in 5–20% of allografts, causing graft failure in
<10%
Variable timing, months to years after transplant
Often present with proteinuria, hematuria, and renal insufficiency
Requires immunofluorescence and ultrastructural examination to diagnose accurately – same criteria for diagnosis as in native kidney
IgA Nephropathy (Fig. 5.14A, B)
♦
Recurs 3 months and later after transplant
♦
25–60% incidence of recurrence
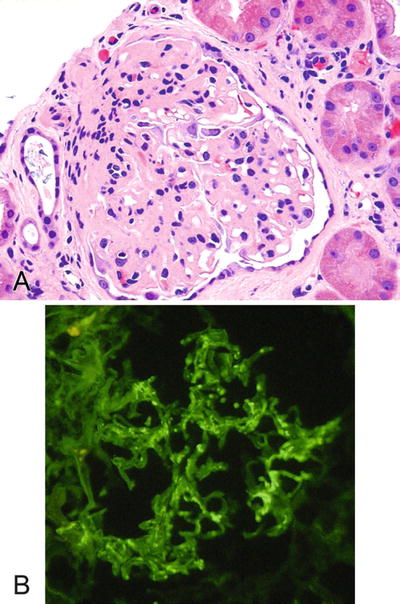
Fig. 5.14.
(A) Allograft kidney with a glomerulopathy from a patient originally transplanted for IgA nephropathy. (B) Immunofluorescence for IgA on kidney indicating recurrent IgA nephropathy.
Necrotizing Crescentic Glomerulonephritis (Wegener Granulomatosis, Microscopic Polyangiitis)
♦
Clinical factors (cANCA, pANCA levels, disease subtype, type of transplant) are not useful for predicting recurrent disease
♦
Recurrence seen in up to 18%
Antiglomerular Basement Membrane (Anti-GBM) Disease
♦
50% recurrence rate when anti-GBM antibodies are in serum
♦
5–15% recurrence rate when serum cleared of anti-GBM antibodies for more than 6 months
Hemolytic Uremic Syndrome
♦
Clinical presentation of recurrence gradual or abrupt
♦
Thrombocytopenia, hemolysis, progressive renal dysfunction
♦
30% recurrence rate with poor graft survival after recurrence
Focal Segmental Glomerulosclerosis
♦
Recurrence is typically early with a mean 14 days after transplant
♦
Proteinuria, hypertension, increased creatinine
♦
20–30% recurrence in first transplant with 40–50% graft loss
♦
75% recurrence in subsequent transplant, if initially lost to recurrent disease
Membranous Glomerulonephritis
♦
~30% recurrence rate
Membranoproliferative Glomerulonephritis
♦
Type I: underlying circulating immunocomplexes usually persist after transplant
20–30% recurrence with 40% graft loss, higher with subsequent grafts
HLA-matched grafts said to be more susceptible to recurrence
♦
Type II: recurs in 50–100% of grafts
Presents after 1 year posttransplant with hematuria and proteinuria
Lupus Nephritis
♦
<10% recurrence rate; all pattern types recur
♦
Usually seen 3 or more years after transplantation
♦
Diabetic changes often recur, typically >1 year after transplant
♦
Present with progressive renal dysfunction typically without or only mild proteinuria
De Novo Glomerulonephritis
♦
Uncommon, when seen, often membranous GN
Drug Toxicity
♦
Cyclosporine or FK506 toxicity
Presents with increased creatinine
Minimal interstitial inflammation
Nodular, new onset, arteriolar hyaline change is suggestive of toxicity (Fig. 5.15)
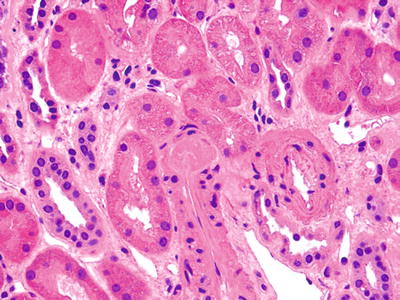
Fig. 5.15.
Small arteriole from an allograft kidney showing intramural hyaline globule consistent with calcineurin inhibitor toxicity. Note the lack of an interstitial infiltrate.
Also vacuolization of smooth muscle cytoplasm in arteriole wall (Fig. 5.16)
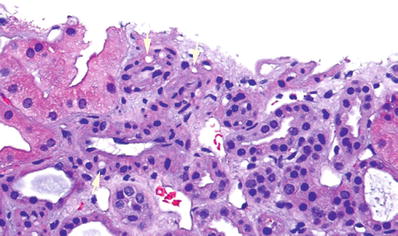
Fig. 5.16.
Small arteriole from an allograft kidney showing vacuolization of the smooth muscle cytoplasm within the wall of the vessel (arrows). This finding is suggestive of calcineurin inhibitor toxicity. Note the lack of an interstitial infiltrate.
♦
Antibiotics
Variable interstitial infiltrate often with eosinophils (Fig. 5.17)
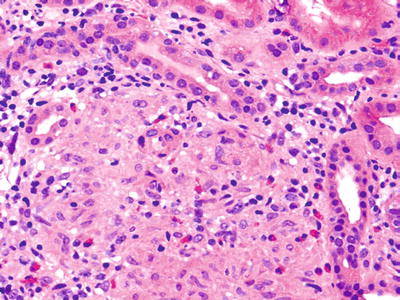
Fig. 5.17.
Interstitium of a renal allograft expanded by a mononuclear infiltrate including numerous macrophages and eosinophils. Some tubular damage is present. The patient presented with creatinine elevation, which resolved with discontinuation of antibiotic therapy – a drug toxicity.
Viral Infections
BK Virus
♦
Small nonenveloped double-stranded DNA virus of the Polyomavirus family
♦
Latent infection present in at least 50% of the population
♦
Usual onset within 3 months posttransplant but can be seen years later
♦
Present with graft dysfunction
♦
Histology shows polymorphous interstitial infiltrate associated with tubulitis mimicking rejection (Fig. 5.18)
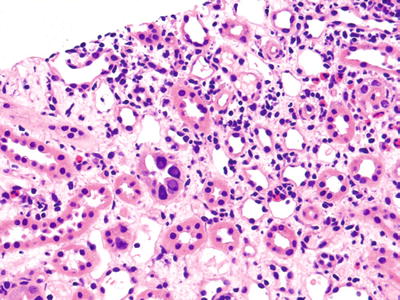

Fig. 5.18.
An allograft kidney with a mild-to-moderate interstitial infiltrate. Two tubules exhibit enlarged nuclei with amphophilic nuclear inclusions very suggestive of BK virus infection. Some tubular damage is present which might be errantly diagnosed as rejection if the viral inclusions were not noted.
♦
Characteristic ground glass to lavender intranuclear inclusions often associated with nuclear enlargement of the tubular epithelium
♦
♦
Treated with decreased immunosuppression
Cytomegalovirus
♦
Typically seen starting 30 days after transplant
♦
Presents with graft dysfunction
♦
Histology shows polymorphous interstitial infiltrate associated with tubulitis may mimic rejection
♦
Characteristic nuclear enlargement with prominent intranuclear and cytoplasmic inclusions
♦
Inclusions may be found in the tubular epithelium, endothelial cells in veins and glomerular capillaries and in stromal cells
Acute Tubular Necrosis
♦
Seen early after transplant, consistent with preservation effect (Fig. 5.20)
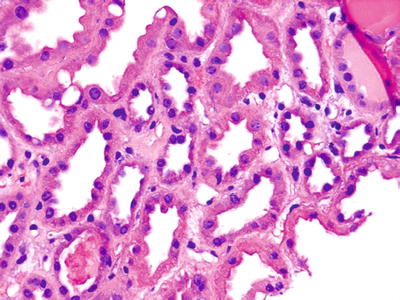

Fig. 5.20.
A kidney allograft several days after implantation showing enlarged tubular nuclei with scattered mitotic figures consistent with recovering ATN.
♦
May be seen late as a manifestation of hypotension or drug effect
Ureter Obstruction/Pyelonephritis
♦
Common, variable timing
♦
Presents with fever, increased creatinine, pyuria
♦
Interstitial infiltrate of neutrophils with edema
Vein Thrombosis
♦
Interstitial edema and marked congestion
Posttransplant Lymphoproliferative Disorder
♦
Patients with kidney transplants have the lowest incidence, <1%
♦
In the allograft the atypical, interstitial, B cell, mononuclear infiltrate with mitotic figures must be differentiated from rejection
♦
EBV often can be demonstrated (EBER)
♦
Treatment is typically decreased immunosuppression
Heart Transplantation
Indication for Transplantation
♦
Chronic severe heart failure due to multiple causes including coronary artery disease, myocardial infarction, myocarditis, congenital malformations, etc
Clinical Considerations
♦
Contraindications to transplantation:
Malignancy
Active infection (HIV)
Amyloidosis
♦
Cadaveric organs are ABO matched to donor
♦
Orthotropic transplant:
Recipient atria (with cavae and pulmonary veins) are anastomosed to donor atria creating slightly enlarged composite atria
Donor aorta above the level of the coronaries anastomosed to recipient aorta
Donor pulmonary artery to recipient pulmonary artery
Rejection
Acute Cellular Rejection
♦
Acute cellular rejection first occurs 7–10 days after transplantation
♦
May present with elevated right-sided heart pressures and/or malaise
♦
No clinical parameter correlates well with rejection, but right-sided pressures and EKG abnormalities may be an indication of rejection
Usually right-sided myocardial biopsy is performed at regular intervals (protocol biopsies) to look for rejection
♦
A minimum of three formalin-fixed myocardial fragments are considered adequate for evaluation
Three H&E-stained levels should be examined
No special stains are required, but special stains may be used as needed, i.e., C4d
♦
Consensus criteria for diagnosis of rejection were initially developed by a multi-institutional panel of experts convened under the auspices of the International Society for Heart and Lung Transplantation (ISHLT) in 1990
After many years of use and follow-up, the system was revised (Table 5.5) to incorporate this experience
Table 5.5.
Revised ISHLT Consensus Grading System for Cardiac Rejection 2005
Grade 0 R
No rejection
Grade 1 R, mild
Interstitial and/or perivascular infiltrate with up to one focus myocyte damage
Grade 2 R, moderate
Two or more foci of infiltrate with associated myocyte damage
Grade 3 R, severe
Diffuse infiltrate with multifocal myocyte damage, +/− edema, +/− hemorrhage, +/− vasculitis
♦
The original cardiac rejection grading system from 1990 was more complex with multiple subgrades
This system is still in use in some institutions, so Table 5.6 is provided to allow comparison with the old system
Table 5.6.
Comparison of the Current and Previous ISHLT Grading Systems
Current classification
Description
1990 classification
Grade 0R
None
Grade 0
Grade 1R
Mild
Grade 1A, grade 1B, grade 2
Grade 2R
Moderate
Grade 3A
Grade 3R
Severe
Grade 3B, 4
It is immediately apparent that the current system has shrunken from four major grades to three and that all the subgrades have been eliminated
The grading system is relatively easy to apply
Focal small infiltrates both perivascular or interstitial with or without minimal myocyte damage are 1R (Figs. 5.21 and 5.22).
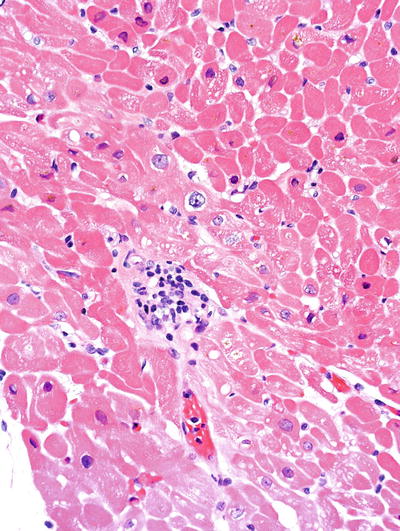
Fig. 5.21.
Allograft heart showing focal small perivascular infiltrates characteristic of grade IR acute cellular rejection. (1a in the original grading system which is still in use in some institutions).
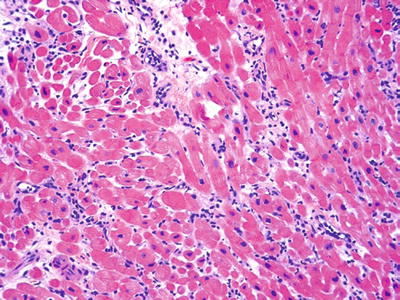
Fig. 5.22.
Small, focal, interstitial lymphocytic interstitial infiltrate 1R (1b in the original system).
In the past a single focus of aggressive appearing mononuclear cells was difficult to classify
It may have represented rejection or penetrating Quilty effect (described below) (Fig. 5.23).
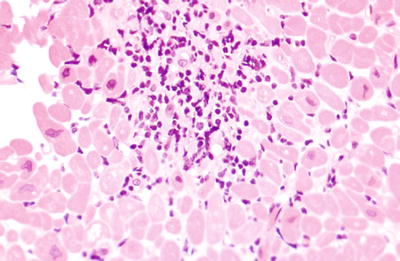
Fig. 5.23.
A single moderately dense lymphocytic infiltrate that was difficult to grade in the old system. One should examine multiple levels though a lesion like this to exclude the possibility of tangentially cut Quilty effect. If no Quilty can be identified, then a diagnosis of grade 1R cellular rejection is appropriate (2 in the original system).
We cut multiple additional levels to see if there is a connection to a surface Quilty lesion; if not, 1R rejection.
Multifocal aggressive infiltrates with focal myocyte damage is grade 2R (Fig. 5.24)
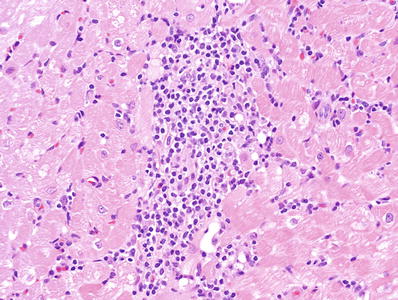
Fig. 5.24.
Moderate to large aggressive infiltrate with myocyte necrosis 2R (3A in the original grading system).
A diffuse inflammatory process with multifocal necrosis or arteritis is 3R (Fig. 5.25)
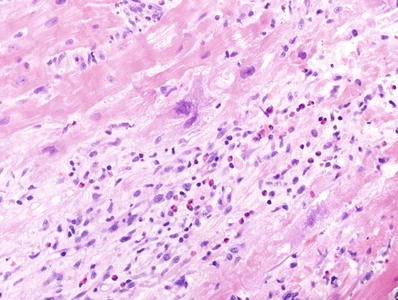
Fig. 5.25.
A diffuse interstitial infiltrate associated with extensive myocyte necrosis, a feature of severe 3R rejection (3B in the original grading system).
Treatment for acute cellular rejection is institution specific
At our institution, in adults, grade 2R and above rejection are treated with increased immune suppression and grade 1R is generally not treated.
Antibody-Mediated Rejection (AMR )
♦
The incidence and diagnosis of humoral rejection are somewhat controversial
♦
Hyperacute rejection has been well described but is uncommon
Graft becomes dusky and ceases pumping immediately after revascularization
Due to preformed antibodies producing microvascular injury, i.e., ABO incompatibility
Histology shows marked congestion and endothelial swelling; fibrin platelet thrombi may be present
♦
Acute/active AMR
Graft is dysfunctional weeks to months posttransplant
May show edema, endothelial swelling, and prominent intracapillary inflammatory cells (neutrophils, macrophages) (Fig. 5.26)
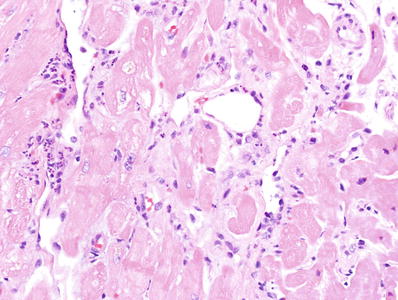
Fig. 5.26.
An endomyocardial biopsy showing dilation of small vessels with swollen endothelium and intravascular neutrophils suggesting humoral rejection.
Immunofluorescence shows immunoglobulin and complement deposition in capillaries
C4d deposition may or may not be seen.
The criteria for a positive C4d are not well established, but staining of more than 50% of the capillaries could be supported by the literature.
Staining in veins and arterioles should not be interpreted as positive.
A recent report employing strict definitions, serology, and immunofluorescence (Fig. 5.27) found a 4% incidence of humoral rejection over a 10-year period; 65% of the cases of humoral rejection were late in onset.
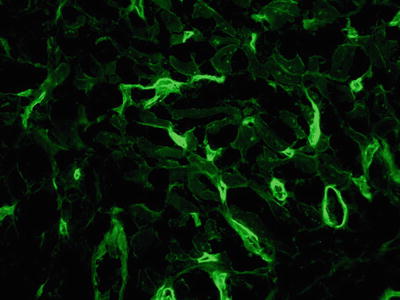
Fig. 5.27.
Immunofluorescence stain for C4d showing diffuse capillary staining consistent with antibody-mediated rejection in the heart. This can also be shown by immunohistochemical methods.
Criteria for the diagnosis of AMR are shown in Table 5.7.
Table 5.7.
Scheme for Evaluating Cardiac Antibody-Mediated Rejection (AMR)
pAMR 0
Negative for AMR: no evidence for AMR by histology or immunopathology
pAMR 1
Histopathologic AMR (pAMR 1 [H+]): findings presented by histology alone (immunopathology negative) Immunopathologic AMR (pAMR 1 [I+]): findings presented by immunopathology alone (histology negative)
pAMR 2
Pathologic AMR: findings presented by histology and immunopathology
pAMR 3
Severe pathologic AMR: histopathologic findings of interstitial hemorrhage, capillary fragmentation, mixed inflammatory infiltrates, endothelial cell pyknosis, and/or karyorrhexis and marked edema
♦
These latest criteria emphasize both histologic and immunologic criteria
Either immunohistochemistry or immunofluorescence is an acceptable method for assessing endothelial injury
CD3 may be a better marker than C4d for AMR.
Intravascular macrophages also strongly correlate with AMR (Fig. 5.28).
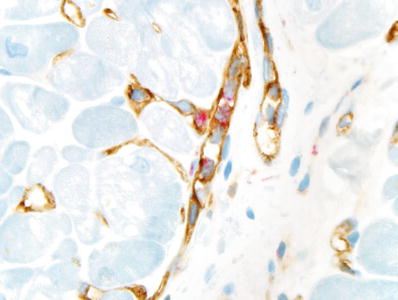
Fig. 5.28.
Immunoperoxidase staining showing CD68-positive macrophages (red) in the lumen of several capillaries (CD31 brown), a histologic feature of antibody-mediated rejection in the heart.
It should be noted that although graft dysfunction is likely a late manifestation AMR, it is usually present
The clinical significance of AMR without graft dysfunction is unclear
♦
DSA is also an important indicator of cardiac AMR
♦
Mixed cellular and humoral rejection may also occur
♦
As noted in other systems, treatment is difficult including increased immunosuppression and plasmapheresis
Transplant Coronary Artery Disease (Cardiac Transplant Vasculopathy/Chronic Rejection)
♦
~10% of heart transplant patients develop clinically significant vessel disease
♦
Risk factors include acute rejection, CMV infection, hyperlipidemia, and glucose intolerance
♦
The etiology of arteriopathy is not completely known, but combination of cellular and humoral injury seems likely
♦
Early: foamy endothelial changes
♦
Late: circumferential fibrointimal proliferation that obliterates the lumen leading to infarction
As the lumen narrows, it predisposes to thrombosis (Fig. 5.29)
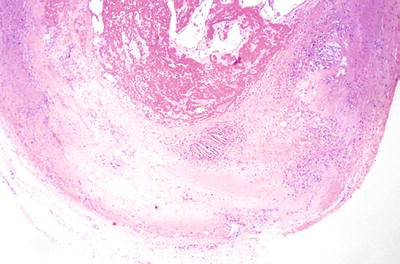
Fig. 5.29.
An epicardial coronary artery from an explanted cardiac allograft showing classic transplant vascular disease of chronic rejection. There is marked fibrointimal thickening of the wall in a concentric fashion with an acute thrombus in the lumen.
♦
Difficult to diagnose without angiogram or echo-angiography
No known treatment
Usually leads to graft failure
Other Biopsy Findings
♦
Focal subendocardial layer of myocyte necrosis in early biopsies consistent with preservation effect (Fig. 5.30)
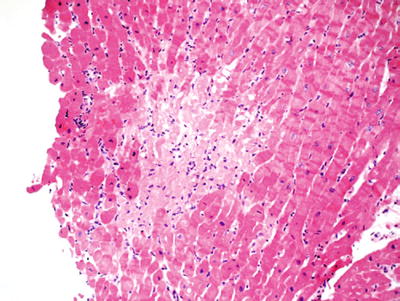

Fig. 5.30.
Protocol endomyocardial biopsy 2 weeks after transplantation showing focal ischemic myocyte necrosis. These small, often subendothelial foci of necrosis are often noted in the first month after transplantation. If this pattern is seen in late biopsies, it is a marker of vascular injury or occlusion.
Goes on to fibrosis with time
The thinner the necrotic layer, the better
♦
Granulation tissue with or without chronic inflammatory cells is an old biopsy site, not rejection (Fig. 5.31)
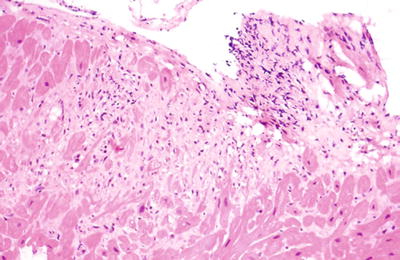

Fig. 5.31.
Fibrosis and granulation tissue consistent with a previous biopsy site in a cardiac allograft.
♦
Quilty effect:
A nodular collection of lymphocytes involving the endocardium (Fig. 5.32)
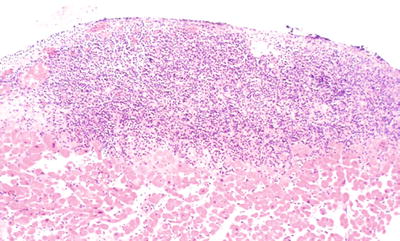
Fig. 5.32.
Classic appearance of Quilty effect . The lesions can be quite variable in size. Small vessels often traverse the infiltrate.
May extend into the endocardium with damage to adjacent myocytes (penetrating Quilty effect) (Fig. 5.33)
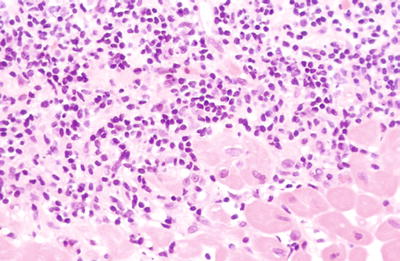
Fig. 5.33.
High power view of the base of the infiltrate seen in Fig. 5.32. Note the myocyte damage at the interface. This is not an indication of rejection. Imagine how this focus would look if cut tangentially.
Small vessels often seen in the infiltrate
Mixture of T and B cells without prominent nucleoli, mitoses, or atypia
Associated with calcineurin inhibitor-based immunosuppression but etiology is not known
Not rejection
Note: if tangentially cut, penetrating Quilty effect may be confused for rejection
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree