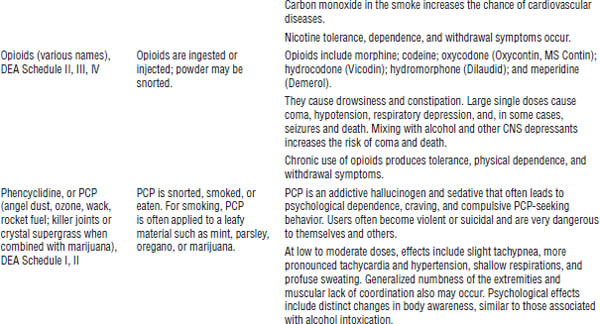
Table 39-1. First Aid for Poisoning Emergencies
Type of emergency | First-aid response |
Inhaled poison | Immediately get the person to fresh air. Avoid breathing fumes. Open doors and windows wide. |
Poison on the skin | Remove any contaminated clothing. Flood skin with water for at least 15 minutes. |
Poison in the eye | Remove contact lenses. Flood the eye with water, pouring it from a large glass 2–3 inches from the eye. Repeat for a total of 15–30 minutes. Do not force the eyelid open. |
Swallowed poison | Unless the victim is unconscious, is having convulsions, or cannot swallow, give a small glassful (2–4 oz) of water immediately. Call a poison control center for advice about whether other actions are needed. |
■ Do not use home remedies such as saltwater, mustard powder, raw eggs, hydrogen peroxide, cooking grease, or gagging.
■ Immediately call 911 or an ambulance if the person is not breathing, has had a seizure, or is unresponsive.
■ For other situations, contact a poison control center immediately to determine whether first aid should be used or whether a poisoning emergency exists.
Decontamination of the Gastrointestinal Tract
The practice of using drugs to decrease the absorption of other drugs from the gastrointestinal tract is in a state of change. For example, ipecac syrup is being abandoned by many as a home- or hospital-based therapy, and its use is primarily at the preference of the consulting poison control center or health care professional. Current recommendations, as well as basic information about the drugs in case they are encountered, are described in this section.
Current recommendations
Ipecac syrup has questionable effectiveness, and its use is generally avoided.
Gastric lavage involves placing a tube into the stomach through a nostril or the mouth and repetitively washing out the stomach contents with water or a saline solution. This method of gastric decontamination is of questionable effectiveness, particularly if it is performed more than 1 hour after ingestion of toxin.
Cathartics such as magnesium citrate are no longer routinely used.
Activated charcoal given orally is often the only treatment necessary if the toxin is adsorbed and the activated charcoal is used within 1–2 hours of ingestion of the toxin.
Whole bowel irrigation can be considered if the toxin is poorly or slowly adsorbed and its presence in the gastrointestinal tract is likely.
Ipecac syrup
Indications and dosage
Ipecac syrup was previously used for general prophylaxis of selected poisonings of expected minor or moderate severity in alert patients. Many clinicians have abandoned it as a prehospital or hospital treatment. In 2003, the American Academy of Pediatrics recommended that ipecac syrup no longer be used routinely as a home treatment for poisoning.
Contraindications
■ The patient is experiencing pronounced sleepiness, coma, or seizures.
■ The patient has ingested caustics, aliphatic hydrocarbons, and fast-acting agents that produce coma or seizures (e.g., tricyclic antidepressants, clonidine, calcium channel blockers, β-blockers, and hypoglycemic agents).
■ Time since ingestion is believed to be 1 hour or more.
Adverse effects
■ Common: Diarrhea, sleepiness, protracted vomiting
■ Uncommon: Mallory–Weiss (esophageal) tears, tracheal aspiration into the lungs
Disadvantage
A disadvantage of ipecac syrup is that emesis and the drug’s relative lack of efficacy complicate administration of other oral therapies.
Activated charcoal
Indications and dosage
This agent is occasionally used to adsorb poisons in an alert or comatose patient. Administer as a slurry by mouth in alert patients or through a lavage tube:
■ Children: 25–50 g
■ Adults: 25–100 g
Contraindications
■ Ingestions of aliphatic hydrocarbons and caustics
■ Absence of patient’s bowel sounds
■ Ingestions of heavy metals (sodium, lithium, iron, or lead) or simple alcohols
Adverse effects
■ Uncommon: Tracheal aspiration, pneumonitis
■ Common: Emesis, soiling of clothes and furnishings
Advantages and disadvantages
■ Advantages: Rapid onset of action, nonspecific action for a wide variety of chemicals, reasonable effectiveness within 1 hour of ingestion
■ Disadvantages: Messy and difficult administration, possible removal of beneficial drugs together with the toxin
Cathartics
Cathartics were previously used as an adjunct to activated charcoal administration to decrease gastrointestinal transit time. Their efficacy is unproved. Fluid and electrolyte disturbances are possible with repeated doses.
Cathartics may contribute to emesis following activated charcoal use.
Agents previously used include magnesium citrate, magnesium sulfate, sodium sulfate, and sorbitol. Some activated charcoal products contain sorbitol mixed in the preparation. The sorbitol concentration varies from brand to brand.
Whole bowel irrigation
Indications and technique
Whole bowel irrigation is generally used to wash out the gastrointestinal tract when using charcoal may be inappropriate (e.g., if iron or lithium was ingested) and the toxin is suspected to be present in the gastrointestinal tract (e.g., when drugs are sustained-release formulations or when the patient ingested illicit drugs packed in condoms). It is not routinely used to treat poisonings except in these unique circumstances.
Use larger volumes of polyethylene glycol electrolyte solutions (e.g., CoLyte, GoLYTELY) than the amounts conventionally used for bowel preparation. Administer by mouth or through a gastric or duodenal tube for treatment of poisoning:
■ Children: 25 mL/kg/h (approximately 500 mL/h) up to 2–5 L
■ Adults: 2 L/h up to 5–10 L
Contraindications
■ Ingestion of caustics or aliphatic hydrocarbons
■ Patients with absent bowel sounds or gastrointestinal tract obstruction
Adverse effects
Few adverse effects have been reported, but limited results are available from which to draw conclusions. Some nausea and vomiting have been reported.
Advantages and disadvantages
■ Advantages: Prompt whole bowel evacuation within 2 hours
■ Disadvantages: Messy procedure because of rectal effluent
Other hospital-based therapies
These therapies include supportive and symptomatic care, multiple doses of activated charcoal (to enhance systemic elimination when appropriate), hemodialysis (to enhance systemic elimination when appropriate), and use of antidotes (to antagonize or reverse toxic effects when indicated).
39-4. Substance Abuse and Toxicology
Substance abuse often leads to acute and chronic toxicity. Table 39-2 describes selected drugs of abuse.
During 2012, 41.5 million Americans age 12 and older (16% of the population) admitted using an illicit drug in the past year, and 2.9 million started using illicit drugs.
Table 39-2. Selected Drugs and Substances of Abuse
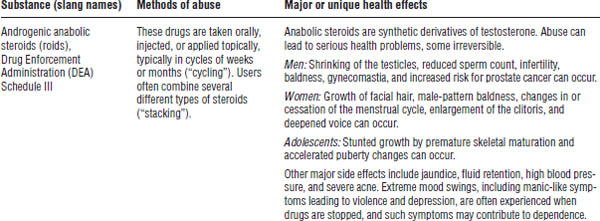



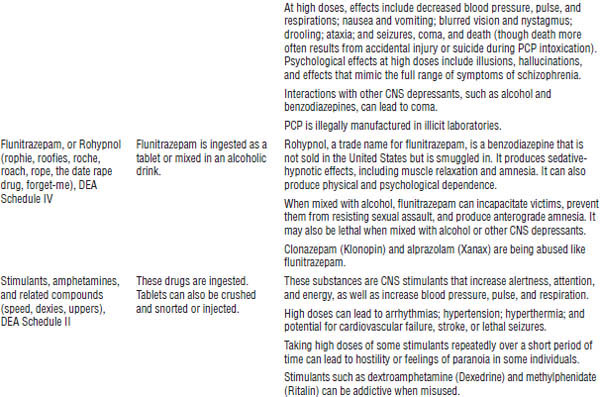
DrugFacts, National Institute on Drug Abuse, National Institutes of Health, 2012; Drug fact sheets, Diversion Control Program, Drug Enforcement Administration, U.S. Department of Justice Web site.
During 2011, 5 million adults were treated in emergency departments for drug-related episodes, with 2.3 million for adverse drug reactions, 2.5 million for drug and substance abuse, and 77,000 children for unintentional poisonings.
Management of the acute condition generally follows the same guidelines as those for management of poisonings and overdoses. A challenge in treating patients during acute drug overdose is determining the possible agents taken and possible adulterants (e.g., talc, strychnine, other drugs) or contaminants.
Chronic abuse can foster dependence, which often leads to withdrawal symptoms when the patient stops using the drugs. Detoxification programs, long-term behavioral counseling, and drugs to produce aversion or substitution to drug-taking behaviors are often needed.
39-5. Antidotes
Role of Antidotes
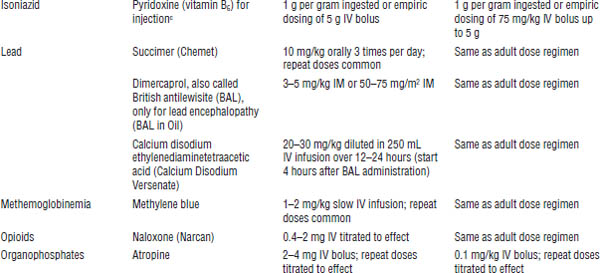
An antidote counteracts or changes the nature of a poison. Few antidotes are available relative to the large number of potential poisons. Table 39-3 lists antidotes that are commonly used in the treatment of a patient with a poisoning or an overdose.
Many hospitals have an insufficient stock of antidotes. The pharmacy and therapeutics committee of a hospital should regularly review the inventory of antidotes that are stocked at the hospital.
Selected Antidotes
Acetylcysteine
Acetylcysteine is available under the trade names Mucomyst (10, 20% oral solution) and Acetadote (20% for injection).
Uses
Acetylcysteine is used to treat acute acetaminophen overdose. An unapproved indication is to treat adverse reactions to drugs and diagnostic agents that may produce free radicals as part of the adverse reaction; the dosage regimen is unique to the particular application.
Mechanism of action
Acetylcysteine protects the liver from the toxic effects of an acetaminophen metabolite by supplying a surrogate for glutathione to aid in metabolism of the reactive metabolite. Other mechanisms are also proposed, which include providing sulfate for acetaminophen metabolism and minimizing the formation of free radicals.
This agent may be useful in minimizing hepatotoxic injury once it has begun. It also may aid in cases of fulminant hepatic failure.
Indications
Acute overdoses of acetaminophen produce a reactive metabolite that leads to hepatotoxicity (jaundice, coagulopathy, hypoglycemia, hepatic failure, hepatic encephalopathy, hepatorenal failure). Symptoms become evident 1–2 days after ingestion.
Acetylcysteine can prevent or minimize hepatic injury if given early. For best results, administer within 10 hours of ingestion of acetaminophen overdose. It is minimally effective when started 24 hours after ingestion.
The need for therapy is determined by obtaining a serum concentration of acetaminophen at least 4 hours after ingestion (and within 24 hours) and plotting it on the acetaminophen nomogram to determine whether there is a risk for hepatotoxicity.
Contraindications
Use of acetylcysteine is contraindicated if there is a known hypersensitivity to the drug.
Adverse effects
With oral administration, nausea and vomiting are common.
With intravenous (IV) administration, anaphylactoid reactions (rash, hypotension, wheezing, dyspnea) have been reported. Acute flushing and erythema may occur during the first hour of infusion and typically resolve spontaneously.
Dosage
Table 39-3 gives dosage information on drug products for oral or IV administration available in the United States.
Atropine
Uses
Atropine is used in cases of organophosphate (including chemical terrorism nerve agents) and carbamate anticholinesterase insecticide poisoning.
Mechanism of action
Atropine is an anticholinergic agent that competitively inhibits acetylcholine at muscarinic receptors. It has little effect on nicotinic receptors in cases of organophosphate insecticide poisoning.
Table 39-3. Commonly Used Antidotesa
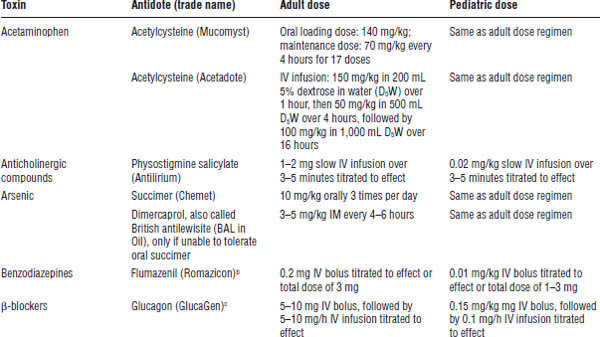

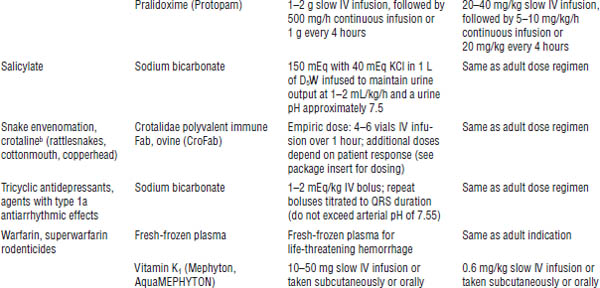
Based on American College of Emergency Physicians, 1999.
IM, intramuscular.
a. The table lists common antidotes that may need to be used emergently for patients presenting with acute toxic ingestion or dermal or inhalation exposure. Dosages are derived from standard texts and references and are given as convenience references. These should not be considered specific treatment guidelines; consult appropriate resources.
b. Potential risks may exceed the benefits because of precipitation of intractable seizures.
c. Off-label indication for use.
d. Information updated by author.
Indications
■ For control of pulmonary hypersecretion, atropine is given in repeated doses intravenously until secretions have decreased. Atropinization may have to be maintained for hours to days.
■ For control of bradycardia, atropine is given until the heart rate increases to an acceptable rate or until a need for alternatives is indicated.
■ Nontoxicologic indications for atropine include premedication to anesthesia induction (for antisecretory effects) and ophthalmic mydriasis and cycloplegia.
Contraindications
There are no contraindications in cases of insecticide poisoning. Contraindications for other indications include the following:
■ Hypersensitivity to atropine or anticholinergics
■ Narrow-angle glaucoma
■ Reflux esophagitis
■ Obstructive gastrointestinal disease
■ Ulcerative colitis or toxic megacolon
■ Obstructive uropathy
■ Unstable cardiovascular status in acute hemorrhage or thyrotoxicosis
■ Paralytic ileus or intestinal atony
■ Myasthenia gravis
Adverse effects
Exaggeration of anticholinergic effects (e.g., tachycardia, hypertension, sedation, hallucinations, mydriasis, changes in intraocular pressure, warm red skin, dry mouth, urinary retention, ileus, dysrhythmias, seizures) can occur.
When large doses of atropine are used, the agent should be free of preservatives because agents such as benzyl alcohol or chlorobutanol can produce their own toxicity.
Dosage
For bronchorrhea and bronchospasm from organophosphates or carbamates, the adult dose is 2–5 mg (pediatric dose is 0.05 mg/kg) slowly administered intravenously. This dose is repeated at 10- to 30-minute intervals until bronchial hypersecretion is resolved. Severe poisonings may require up to 100 mg over a few hours to several grams over several weeks. If atropinization is required for several days, continuous atropine infusion may be used (rates of 0.02–0.08 mg/kg/h are recommended).
For symptomatic bradycardia from mild poisonings, the adult dose is 1 mg (pediatric dose is 0.01 mg/kg) intravenously. For moderate to severe poisonings, adult doses increase to 2–5 mg (pediatric doses are 0.02–0.05 mg/kg) and should be repeated every few minutes until the heart rate increases.
These doses for atropine are higher than those routinely used for indications not related to organophosphate poisoning, such as bradycardia after a myocardial infarction.
Digoxin immune Fab (Digibind, DigiFab)
Uses
Digoxin immune Fab is used to treat life-threatening acute or chronic digoxin poisoning.
Some cross-reactivity with digitoxin and other digoxin-like compounds (digitalis, foxglove, lily of the valley, and bufadienolide from cane frogs) can occur.
Mechanism of action
Digoxin immune Fab binds digoxin in plasma, promotes redistribution from tissues, and enhances elimination in the urine. The digoxin bound to digoxin immune Fab is inactive. Each 40 mg (1 vial) binds 0.6 mg of digoxin.
Digoxin immune Fab is a monovalent, digoxin-specific, antigen-binding fragment (Fab) that is produced in healthy sheep.
Indications
Chronic digoxin toxicity typically begins with nausea, vomiting, diarrhea, fatigue, confusion, blurred vision, diplopia, and the observation of white borders or halos around dark objects. Deterioration of renal function, hypokalemia, or drug interactions often leads to toxicity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree