Peripheral neuropathic conditions treated with Topical capsaicin
Postherpetic neuralgia (PHN)
Painful diabetic polyneuropathy (PDPN)
HIV-associated distal symmetrical polyneuropathy (HIV-DSPN)
Persistent postsurgical pain
4.2 Neuropathic Pain
Whether noted by clinician or patient, neuropathic pain can be one of the most disabling and challenging medical conditions to effectively manage. This is in part due to the diverse and poorly understood pathophysiological drivers that are associated with persistent neuropathic pain. Of the known sources of painful neuropathic pain, this chapter will focus on a subgroup of those of peripheral origin or manifested in a peripheral site of disease that have shown to be responsive to topical capsaicin. These include infectious (herpes zoster, HIV, metabolic (diabetes), and nerve entrapment/surgical/trauma). Although it is uncertain what specific property these diverse disease states or lesions share that engender the development of persistent painful neuropathic conditions, by definition, pain arising as a direct consequence of a lesion or disease affecting the somatosensory system either peripheral or central, now serves as the current definition of neuropathic pain by the International Association for the Study of Pain—Special Interest Group on Neuropathic Pain (NeuPSIG).
4.2.1 Pathways and Mechanisms
4.2.1.1 Nociceptors: The Site of Capsaicin Action
Since the early observations of Sherrington, who believed that the experience of pain was based on nerves that responded to specific types of noxious stimuli that cause tissue damage, the concept of “nociceptive nerves” and later the term “nociceptor” emerged to describe what we now refer to as primary afferent nociceptors (Sherrington 1906). Nociceptors represent that portion of the peripheral nervous system that is specialized for the detection of noxious stimuli. One of the principle benefits provided by nociceptors is their rapid detection of impending or actual tissue injury. Nociceptors accomplish this by being a part of an integrated system or “pain pathway” beginning with peripheral nociceptive terminals that function to detect multiple noxious stimuli (transduction), the relay of these signals to the central nervous system through the conduction of action potentials (transmission) and finally their interpretation as a harmful or unpleasant experience (perception) (Fields 1990).
Within the peripheral nervous system, somatosensory detection of tissue damaging stimuli (thermal, mechanical, chemical) begins at the peripheral terminals of primary afferent nociceptors whose cell bodies reside primarily in the trigeminal (V) and dorsal root ganglia (DRG). In addition, cranial nerves V (innervation of the majority of the face, conjunctiva, mouth, and dura mater) as well as cranial nerves VII, IX, and X (innervation of the skin of the external ear, and mucous membranes of the larynx and pharynx) also participate in nociception (Carpenter 1985). Likewise, nerve terminals derived from nociceptors residing in spinal dorsal root ganglia (cervical, thoracic, and lumbar) innervate the somatotopic dermatomes of the skin and underlying tissue. Nociceptors derived from dorsal root ganglia then send central processes to laminae I, II, and V of the dorsal horn of the spinal cord and following synaptic connection with second-order dorsal horn neurons, nociceptive information is relayed to higher centers of the brain.
Detection of noxious stimuli of the skin and underlying deep tissues (somatic) has been divided into three modalities: noxious thermal, mechanical, and chemical. The behavioral and physiological responses following the application of one of these three painful modalities have served as the cornerstone for a classification scheme of nociceptors. Within this classification scheme, the threshold for evoking the sensation of pain has been determined in human volunteers with certain external forces applied to the skin such as noxious thermal stimuli (temperatures >43–45 °C) or intense mechanical stimuli. Criteria for detection of noxious chemical stimuli have also been applied and rely on the sensation of pain in response to certain compounds such as capsaicin, the pungent principle ingredient in hot chili peppers and the therapeutic focus of this chapter (Fields 1990).
Primary afferent nociceptors have been further classified based on their axon diameter, conduction velocity, degree of myelination, and more recently, cross-sectional area of neuronal soma. The axons of primary afferent neurons fall into three distinct groups, Aß (large-diameter, 6–22 μm, heavily myelinated with fast conduction velocities (CV) of 33–75 m/sec), Aδ (diameter 2–5 μm, thinly myelinated with CV 5–30 m/sec), and C fibers (diameter 0.3–3 μm, unmyelinated with CV of 0.5–2 m/sec) (Bessou and Perl 1969). Nociceptors activated by multiple noxious stimuli are referred to as “polymodal nociceptors.” (McMahon and Koltzenburg 1990) Included in this category are C fiber type mechano-heat nociceptors and at least two types of A fiber type nociceptors: mechano-heat Type I (high heat threshold >49 °C) and mechano-heat Type II, (heat threshold ~43 °C) (Fields 1990). Finally, high threshold mechano-nociceptors that fail to respond to thermal stimuli have been characterized for both C and A fiber types as well as for nociceptors that respond only to noxious chemical stimuli.
The sensation of peripheral neuropathic pain arising from peripheral sites of pathology has been described as arising from both unmyelinated C-type (slowly conducting) nerve fibers associated with sensations of dull, aching, burning, and poorly localized pain as well as thinly myelenated Aδ nerve fibers which are more rapidly conducting and signal sensations of sharp, stabbing, and often well-localized pain. However, despite this elegant classification of nociceptor subtypes, discharge patterns of polymodal nociceptors do not precisely correlate with stimulus-induced pain sensation (Adriaensen et al. 1984). Therefore, central processing of nociceptor impulses must be required for the discrimination of painful sensations. Although not proven, one may hypothesize that the selective destruction and/or functional silencing of a subset of polymodal nociceptors following topical capsaicin treatment, as discussed below, could disrupt the input of peripheral neuropathic pain signal processing of polymodal nociceptors.
Nociceptors also have the ability to adjust their sensitivity following repetitive noxious stimuli or tissue/nerve injury. Sensitization encompasses an increase in spontaneous nociceptor activity, a lowered threshold for activation, and an increase in action potential firing after suprathreshold stimuli (Fields 1990). Together with plasticity changes in the dorsal horn of the spinal cord, sensitization of nociceptors contributes to hyperalgesia. Nociceptor modulation is complex and multiple pathways exist to both detect noxious stimuli and modulate transducing element sensitivity. Under neuropathic conditions this complexity is increased, driven by overlapping biochemical processes—some common to both tissue and nerve injury. Examples of changes more prominent to experimental neuropathic pain models arising from peripheral nociceptor sensitization include increased small-afferent signaling arising from distal sprouting of injured nerves and aberrations in nociceptor channels/receptor expression (sodium and calcium channels, Nerve Growth Factor/NGF receptor-TrkA) in injured and uninjured (adjacent) sensory neurons.
One signaling molecule long associated with experimental neuropathic pain is the trophic factor “nerve growth factor” (NGF). Since its identification by Levi-Montalcini and Calissano, NGF has been distinguished from other neurotrophin family members (brain-derived neurotrophic factor NT-3, and NT-4/5) as being essential for normal nociceptor development and function (Koltzenburg 1999; Lewin and Mendell 1994; McMahon et al. 1995). NGF is synthesized and secreted by a wide variety of tissues including Schwann cells located within sensory ganglion and importantly, in the end-target tissues of nociceptive terminals—epidermal fibroblasts and keratinocytes. NGF is intimately involved in maintaining and modifying the phenotype of the nociceptor population. Adult sensory neurons lose their dependency on NGF for survival but retain expression of its high-affinity receptor TrkA primarily on the small-diameter primary afferent nociceptors (C and Aδ) (Koltzenburg 1999). Tissue and nerve injury are associated with increased NGF production and content at the site of the injury, serving as the driving signal for the associated pain and hyperalgesia (Woolf and Costigan 1999). Therefore, long-term exposure of nociceptive terminals to increased levels of NGF can result in long-term phenotypic changes in the repertoire of nociceptive transducing elements such as the capsaicin receptor. Such changes may lead to aberrations in pain signaling and in turn, may represent a molecular template for sustained peripheral neuropathic pain. Although the focus of this chapter is on peripheral mechanisms of neuropathic pain and its treatment with topical capsaicin, other more central changes associated with models of neuropathic pain also include the loss of the blood brain barrier integrity surrounding the spinal cord, allowing migration of non-neuronal inflammatory cells into the dorsal horn (sensory) of the spinal cord and the DRG. Other changes associated with experimental models of neuropathic pain include activation of dorsal horn microglia that are known to be associated with chronic pain (Watkins et al. 2001).
4.2.2 Assessment
Neuropathic pain often goes unrecognized and therefore is under-reported being unsuccessfully treated with agents such as non-steroidal anti-inflammatory drugs (NSAIDS) and/or acetaminophen (Gore et al. 2007). Although there is no “gold standard,” pathognomonic sign or symptom for the diagnosis of neuropathic pain, a focused history and sensory exam can often provide clinicians the critical insight for early recognition and subsequent treatment. A combination of signs (hypoesthesia, hyper/hypo-algesia, heat/cold hyperalgesia, allodynia) and symptoms (paraesthesias, sensation of burning, and/or shooting pain) together with the appropriate clinical context increases the likelihood of a reliable diagnosis of neuropathic pain (Haanpaa et al. 2009).
To assist the clinician, standardized screening tools have been developed to provide the practitioner a reliable and importantly, validated approach to forming an accurate diagnosis. Two such tools are the “Leeds assessment of neuropathic symptoms and signs” (LANSS) (Bennett 2001) and the “Douleur Neruopathiques 4 questions” (DN4) (Bouhassira et al. 2005). Although a clinical history may provide a high degree of suspicion about whether a particular patient indeed is suffering from pain of peripheral neuropathic origins, the incidence of neuropathic pain varies geographically. Whereas patients in developing countries with high rates of HIV or trauma due to war may be predisposed to neuropathic pain from these processes, patients from developed countries may be more likely to develop neuropathic pain as a consequence of diabetes or a herpes zoster infection predisposing to PHN (Haanpaa et al. 2009).
4.2.3 Treatment of Neuropathic Pain
Evidence-based guidelines for the treatment of painful neuropathic conditions continue to gain strength as additional randomized controlled trials are successfully completed. Expert opinion in the form of guideline recommendations have emerged and in many cases have been updated from societies dedicated to the evidence-based management of neuropathic pain such as NeuPSIG (Special Interest Group on Neuropathic Pain of the International Association for the Study of Pain) (Dworkin et al. 2010, 2007). Therefore, guidelines intended to recommend any therapy as a “first-line” treatment for a particular type of neuropathic pain require that efficacy has been established in multiple randomized clinical trials (RCT) (Oxford Center for Evidence-based Medicine, Grade A) and that these recommendations are consistent with the provider’s experience/patient population. Unfortunately, few neuropathic pain therapies including those involving the topical application of capsaicin, fulfill such criteria. Nevertheless, an evolving list of first-line medications have been recommended that include: antidepressants with both norepinephrine and serotonin reuptake inhibition, calcium channel alpha 2 delta ligands (gabapentin and pregabalin), and topical lidocaine (Dworkin et al. 2010). Second-line therapies (which may be considered “first-line” under certain circumstances) include tramadol and opioid analgesics. Finally, so-called “third line” therapies include other anticonvulsants and “low-dose” capsaicin creams, as high-dose capsaicin-based patch studies were just emerging as these guidelines were reported. These recommendations are in line with other international expert groups (Attal et al. 2010). Among a collection of European countries that participated in comparison of their clinical practice guidelines on the treatment of neuropathic pain in cancer patients, all responded that the use of amitriptyline was a first-line recommendation (Piano et al. 2014). Second, the use of gabapentinoids was recommended. Within this report, the clinical practice guidelines across nine countries also included whether the use of capsaicin-containing plasters should be recommended for the treatment of conditions of “local (peripheral) neuropathic pain”, presumably having a restricted pattern of distribution (dermatomal or non-dermatomal). Although four of the countries did not provide data, the remaining five countries all recommended that capsaicin-containing plasters should be utilized for the treatment of neuropathic conditions (Piano et al. 2014).
Therefore, providers are faced with a range of choices from pharmacologic therapies of nonopioid and opioid agents, adjuvant analgesics, topical preparations, and interventional techniques such as neuroblockade and intraspinal infusions to recently advancing neurostimulatory techniques. Unfortunately, there is even less evidence in support of interventional approaches to manage pain from neuropathic conditions refractory to pharmacologic interventions. Due to the paucity of RCT data in this area, only weak recommendations so far exist that include the use of epidural injections for herpes zoster, epidural steroid injections for radiculopathy, spinal cord stimulation (SCS) therapy for failed back surgical syndrome, and SCS for complex regional pain syndrome type 1 (CRPS type 1) (Dworkin et al. 2013). More invasive-ablative techniques are also sometimes used under conditions of compassionate care for patients with progressive malignant disease driving neuropathic symptoms (Kanpolat et al. 2009; Meyerson 2001; Turnbull et al. 2011) although these can also be associated with significant risks or unmasking other painful sensations.
As we await additional randomized clinical trials, a step-wise approach for the treatment of neuropathic pain is recommended where a combination of proven medications may represent a superior therapeutic plan. Nevertheless, clinical trials investigating the use of such combination therapies have yet to fully emerge. Just as one considers the application of multiple pharmacologic agents to a particular condition, patients suffering from chronic neuropathic pain will likely benefit from a multimodal or multidisciplinary approach to their pain management. Importantly, multidisciplinary treatment programs compared with conventional treatment are effective in reducing the intensity of the pain reported by the patient for a period of four months to one year (evidence category A2). Although multimodal therapy has been shown to be efficacious for a number of conditions, its superiority over uni-modal therapies has not yet been fully supported in the literature due to the absence of sufficient high quality randomized controlled trials (category D evidence). Nevertheless, it is the recommendation of the American Society of Anesthesiologists—ASA task force in 2010 on practice guidelines for chronic pain management, that “a multimodal strategy be part of the treatment plan for patients with chronic pain” (2010). Moreover, such a multi-modal approach should ensure early recognition and treatment of psychosocial maladies that are critical for long-term success in any treatment plan and goal to improve functional status and overall quality of life.
4.3 The Action of Capsaicin on Primary Afferent Nociceptors
It has long been appreciated that initial applications of capsaicin are painful, but paradoxically, repeated application produces a topical analgesic effect. It has been proposed that a series of overlapping capsaicin-induced effects that include: desensitization, nociceptor dysfunction, neuropeptide depletion, (Cao et al. 1998; Yaksh et al. 1979), and nociceptive terminal destruction (Robbins et al. 1998; Simone et al. 1998) constitute critical elements producing pain relief. However, several aspects of topical capsaicin treatment appear to limit its overall effectiveness and application in clinical practice. The first is the requirement for repeated capsaicin application (up to 4–5 times daily) to establish and maintain an adequate degree of analgesia. Repeated use of capsaicin-containing topical creams leads to the loss of epidermal nerve fibers that can be detected as soon as 3 days following repeated application. In fact, after 3 weeks of capsaicin treatment on the volar forearm 4 times daily, there was an approximately 80 % reduction in epidermal nerve processes. Loss of the epidermal fibers (see Fig. 4.1) was concordant with a reduction in painful sensation to noxious heat and mechanical stimuli (Nolano et al. 1999). Similar findings were observed when capsaicin was injected subcutaneously in volunteers (Simone et al. 1998).
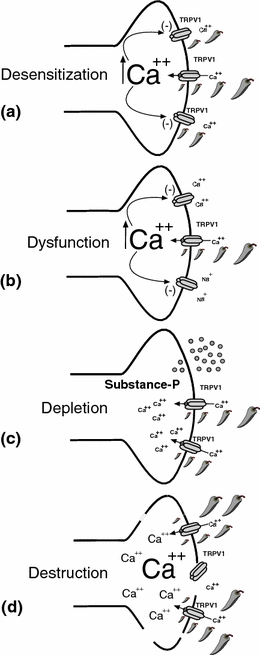

Fig. 4.1
Topical capsaicin-mediated analgesia. Topical application of capsaicin to the skin can produce concentration, dosing interval, and time-dependent changes to underlying sensory nerve terminals expressing the capsaicin receptor (TRPV1). As illustrated in panels (a–d), functional (a–c) and subsequently structural (d) changes in the TRPV1-expressing nociceptive terminals occur as a result of the magnitude and duration of TRPV1 activation. Long-term analgesia may arise from selective destruction and/or functional silencing of a subset of polymodal nociceptors following topical capsaicin treatment disrupting central nociceptive input from peripheral neuropathic pain signal processes. a Desensitization is a calcium-dependent phenomenon where application of capsaicin leads to a decrease in inward current response during continued capsaicin application. When capsaicin is applied at repeated intervals, each subsequent response becomes smaller and is often referred to as tachyphylaxis. TRPV1 may become refractory to the effect of endogenous inflammatory mediators and intracellular secondary messengers. b Repeated or prolonged application of capsaicin can also produce nociceptor dysfunction. Under this condition, which is dependent on the influx and/or excess of store-released calcium, other pain-transducing receptor–channels are inactivated. This may explain analgesic effects beyond the known function of TRPV1. c Depletion of neuropeptides (Substance-P, CGRP) from nociceptive terminal is evoked by capsaicin (low- and high-dose) or repeat applications. Substance-P has been show to play a key role in facilitating nociceptive neurotransmission in the dorsal horn of the spinal cord. d Destruction of TRPV1-expressing nociceptive terminals has been the most reliable marker correlating the application of capsaicin with analgesia
Yet in other clinical studies performed to better quantify the effects of low-dose capsaicin on expression of TRPV1 and neuropeptides in human nociceptive terminals, control human skin biopsies showed abundant immunoreactivity to the neuropeptides SP (substance-P) and CGRP (calcitonin gene related peptide). After 1 day of repeated topical application of capsaicin (five times daily) at a concentration of 0.025 %, a diminution of SP and CGRP immunoreactivity in nerve fibers was observed (Stander et al. 2004). Continuous application of capsaicin for 24 days, 1 and 8 months, respectively, resulted in a decrease of SP and CGRP expression in superficial small nerve fibers of the papillary dermis, whereas, in nerve fibers of the deep dermis, the content of neuropeptides was unchanged. In contrast, the distribution and intensity of TRPV1 staining in nerve fibers and appendage structures was not changed as compared to untreated skin. Seven and 14 days after discontinuation of the capsaicin therapy, immunoreactivity to SP and CGRP was again detectable in small papillary nerve fibers. We, and others, hypothesize that capsaicin directs a dose-dependent effect, with low-dose capsaicin treatments associated with loss of neuropeptides in sensory terminals whereas the repetitive and/or highest capsaicin dosing being the most effective in directing the destruction of nociceptor terminals. What properties of nociceptors allow for their selective inactivation/destruction following capsaicin application?
With the isolation of a cDNA clone encoding a capsaicin-activated ion channel in 1997, the molecular basis of the capsaicin receptor, transient receptor potential cation channel subfamily V member 1 (TRPV1) was finally realized (Caterina et al. 1997). Termed TRPV1, it encodes a nonselective cation channel subunit of approximately 95 kDa that is highly expressed in the small-diameter sensory neurons of dorsal root, trigeminal, and vagal ganglion. Its structure mostly resembles that of members of the Kv 1.2 and store-operated channel family (Caterina et al. 1997). The TRPV1 subunit spans the plasma membrane six times, containing large N- and C-terminal intracellular regions, and is proposed to form tetrameric and/or heteromeric channel complexes (Eilers et al. 2007; Kedei et al. 2001; Kuzhikandathil et al. 2001). It is activated by capsaicin on the intracellular surface in a dose-dependent manner. Once activated, TRPV1 allows for the depolarization of the nociceptor through the conduction of cations. However it is not selective for monovalent cations but rather it preferentially conducts calcium through its channel pore, resulting in an increase in intracellular calcium and cellular depolarization. Thus, TRPV1 can be considered a cellular sensor and when expressed on sensory neurons, can confer specialized properties such as the detection of noxious stimuli and as explained below, detects changes in endogenous signaling molecules associated with tissue injury and inflammation.
Nerve and tissue injury result in the production and release of multiple inflammatory products that have been characterized and identified to directly activate TRPV1. These include products of inflammation and tissue injury such as NGF (Koltzenburg 1999; Mendell et al. 1999) as well as anandamide, the endogenous ligand for the cannabinoid receptor (CB1) (Smart et al. 2000; Julius and Basbaum 2001). Products of the lipoxygenase pathway of arachidonic acid, 12-(S)-hydroperoxyeicosatetranoic acid (12-(S)-HPETE), and leukotriene B4, (LTB4) have also been found to activate TRPV1 in vitro (Shin et al. 2002). It has been reported that TRPV1 is expressed on large and small cutaneous nerve fibers in the human dermis and at the epidermal–dermal junction, while intraepidermal nerve fibers only occasionally stained for TRPV1. A similar staining pattern of TRPV1 immunoreactivity was also described in rat skin (Guo et al. 1999). Importantly, TRPV1 and SP are also co-localized in human cutaneous nerve fibers confirming previous reports in rat and mice. Therefore, beyond the ability of capsaicin to disrupt the function of polymodal nociceptors expressing TRPV1, capsaicin-induced inactivation could also result in blockade of endogenous activation of TRPV that is associated with pathophysiologic conditions giving rise to neuropathic pain symptoms.
4.3.1 Painful Neuropathic Conditions Treated with Topical Capsaicin Preparations
4.3.1.1 Overview
Early meta-analysis that included patients suffering from diabetic neuropathy and osteoarthritis concluded that topical capsaicin improved pain when compared with a placebo (Zhang and Li Wan Po 1994), the analysis includes a number of uncontrolled and/or underpowered trials, a concern that has weakened their influence on changing clinical practice over time. Moreover, if one applies a more “rigorous” standard for clinical trials (as exists presently) on trial data prior to 2004, topical capsaicin (0.025 or 0.075 %) showed poor to moderate efficacy in the treatment of either musculoskeletal or neuropathic symptoms (Mason et al. 2004). Coupled with one-third of these study patients experiencing mild to moderate adverse effects such as erythema, irritation, and transient increase in pain, (see Table 4.2) enthusiasm for widespread use of these agents in the absence of concurrent local anesthetic pretreatment appeared to plateau. Therefore, such treatments were considered for so-called “nonresponders” rather than as a first-line treatment option. To obtain improved patient acceptance and analgesic efficacy using capsaicin-based creams, therapeutic trials have progressively shifted from repeated applications of a combination of local anesthetic pretreatment followed by low-dose capsaicin (Turnbull et al. 2011) to potentially a single application of high-dose topical capsaicin patch. This shift, in part, owes its origin to a case series that included 10 patients suffering from intractable lower extremity pain with neuropathic features. Application of capsaicin (5–10 %) under regional anesthesia resulted in a wide range of posttreatment pain relief (Robbins et al. 1998).
Table 4.2
Topical capsaicin formulations
Low concentration topical | High concentration topical |
|---|---|
Over the counter | Prescription |
Multiple formulations including cream, liquid, patch | Patch |
Generally at or below 0.15 % capsaicin | 8 % capsaicin |
Applied by patient at any location | Applied by medical professional in an appropriate clinical venue |
Application repeated multiple times daily for anywhere from weeks to indefinite time period | Applied once for one hour, repeated as needed at three month or greater intervals |
Does not require pretreatment to the application area | Requires pretreatment with topical local anesthetic to the application area |
Therefore, the report of Robbins et al. suggested an alternative approach, a single application period of a high-dose capsaicin rather than the onerous task of repeat daily applications of low-dose formulations that are associated with a high dropout rate. Later, a high concentration capsaicin patch (8 %) was devised and its application to the skin for a period of 1–2 h produced longer term changes in epidermal nerve fibers that included loss of staining and reduction of heat sensitization. This illustrated that a short-term application of a high concentration of capsaicin can mimic those changes previously seen under repeat application (3–5 times/day for 1 week) of lower concentration capsaicin cream (Malmberg et al. 2004). Subsequently, a randomized double-blinded study for the treatment of PHN using a 1-hour application of a high-dose capsaicin (8 %) patch was found to provide significant pain relief between study weeks 2–12 (Backonja et al. 2008). However, just as repeated application of low-dose capsaicin creams has found limited acceptance by patients due to application-induced burning and/or irritation, application of a high-dose capsaicin patch must also overcome a similar therapeutic barrier. To address this additional therapeutic barrier, high-dose capsaicin patch administration requires a unique application protocol as compared with low-dose breams and a health care provider (see Table 4.2). Pilot studies indicated that pretreating the proposed application area with 4 % lidocaine jelly for one hour was reported to provide “acceptable” side effects following application of high-dose capsaicin patch, though such treatment was commonly associated with localized pain and erythema (Backonja et al. 2008).
4.4 Safety and Tolerability of High-Dose Capsaicin Patch
Beginning with a series of small, open label studies, more rigorous double-blinded randomized controlled studies focused on the safety and tolerability of high-dose capsaicin-containing patches following prior application of a topical local anesthetic. Importantly, it was studied whether the type of local anesthetic influenced the degree of tolerability of the subsequent capsaicin patch application. In a randomized prospective study of 117 patients suffering from a range of peripheral neuropathies including PHN and diabetic neuropathy, using LMX 4, Topicaine, or Betacaine, with endpoints of average pain score at baseline versus at 2–12 weeks, no significant difference in tolerability was found between the various local anesthetics (Webster et al. 2012). Although no serious adverse events (AE) related to high-dose capsaicin patch treatment were reported, 50–59 % of the study patients reported adverse effects (see Table 4.3), which were primarily local, mild to moderate in severity, and resolved within in 1–2 days. Furthermore, analgesic responses were not significantly different between local anesthetic pretreatment groups. Importantly, patients receiving a longer (90-min) patch application were associated with greater procedural discomfort and greater use of periprocedural analgesics without a proportional increase in analgesic effect. Thus a 60-min application period was largely adopted (Webster et al. 2012). Moreover, these results mirror a smaller study with a focus only on patients with PHN treated topically with lidocaine 2.5 %/prilocaine 2.5 % cream for 60 min before receiving a single 60-min application of topical high concentration capsaicin. Maximum mean change in NPRS score was +3.0 observed at 55-min postpatch application with scores gradually declining to near preanesthetic levels within 85 min of patch removal. However, half of the patients received analgesic medication on the day of treatment (Webster et al. 2011). More broadly, analysis of twelve studies of mixed experimental design and patients with various peripheral painful neuropathies (PHN, HIV-DSPN, and PDN) showed that the maximum increase in pain reporting score ranged from 2.3 to 2.8 with no difference in treatment-associated pain among the different neuropathies (Webster et al. 2012).
Table 4.3




Capsaicin application site adverse effects
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


