Fig. 2.1
Hierarchical display of genomic, transcriptomic, proteomic, and metabolomic technologies in biomedical research. TMA technology is an important component of proteomics for high-throughput studies of protein biomarkers
The Proliferation of Microarray Technologies
The development of high-throughput screening technologies has significantly proliferated in the last decade [5–9]. It is now possible to analyze the expression of thousands of genes at once from small quantities of tissue or cell samples [10]. Microarray technologies have pervaded all the medical, veterinary, and biological disciplines. These technologies allow investigators to determine the effects of hormones, cytokines, drugs, and environmental conditions on gene expression levels. The up- or downregulation of subsets of genes in response to the stimulation of interest is then compared with un-stimulated controls. Microarray technology can also be used to help understand cancer development, to improve patient treatment and management, and to identify those predisposed to develop cancer [11, 12]. Microarray expression patterns are currently being integrated with clinical data to identify new biomarkers to predict the potential aggressive behavior of tumors. Protein arrays are also becoming increasingly popular as the equivalent tool in the hands of the proteomics expert. However, proteomic approaches have received less interest from histopathologists and pathologists who are primarily interested in equivalent microscopic and histologic technologies, which will allow them to screen large numbers of tissue specimens for protein expression information and for discovering diagnostic and prognostic correlations.
Tissue Microarrays
Tissue microarray (TMA) technology is a relatively new technique, which allows rapid and simultaneous visualization of molecular targets in hundreds or thousands of tissue specimens [13] (see Fig. 2.2). The molecular targets can be DNA, RNA, or protein depending on the type of probe used for the analysis [14]. This methodology was established in response to an acute need for a reliable high-throughput technique for comprehensive screening of large numbers of human tumors [12, 13, 15]. Figure 2.3 illustrates the steps involved in producing TMAs. High-throughput TMA technology enables the clinical relevance of molecular markers to be assessed simultaneously in multiple tissue specimens [14]. The widespread adoption of TMAs in research laboratories worldwide has replaced the conventional and frustratingly tedious one-slide-one-section approach, in which individual archival clinical specimens were placed on separate microscope slides, with the ability to assess RNA, DNA, or protein biomarker expression in numerous individual tissue specimens in a single carefully controlled experiment [14]. This technology has found applications in almost all areas of human oncology and clinical medicine, and is significantly accelerating the pace of advances in translational research through more efficient assessment of new biomarkers. This technique has not only allowed pathologists to reevaluate the use of existing tumor markers but has also facilitated clinical practice by linking outcome and response to chemotherapy.
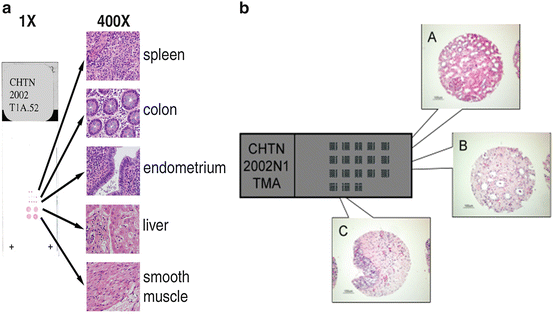
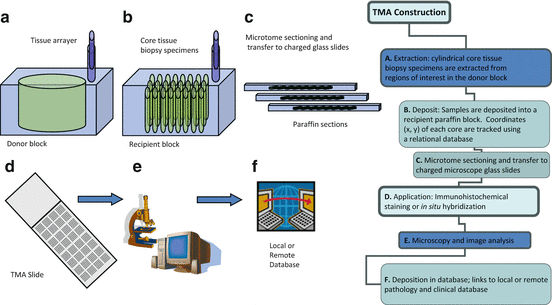

Fig. 2.2
(a) Test microarrays of normal human tissues provide researchers with an inexpensive TMA of a limited number of formalin-fixed paraffin-embedded samples that can be used for optimization of immunohistochemical and in situ hybridization parameters prior to use of more comprehensive tissue microarrays. (b) Example of a high-density CHTN showing hematoxylin and eosin stained cores from normal human kidney cortex (A), medulla (B), and prostate (C)

Fig. 2.3
TMA design and construction . The use of high-density TMAs allows validation of novel protein biomarker discovery and validation across all human tissues by associating TMA data with clinical and pathological data. Using this approach, novel markers of disease pathogenesis may be validated
Basic scientists and clinical investigators have been using multiple tissue Northern and Western blots for many years for studies of gene and protein expression. However, these methods are expensive, laborious, and time-consuming to establish. More importantly these conventional approaches do not permit high-throughput analysis. Proteomics and TMAs are receiving considerable interest from scientists who are increasingly embracing these new technologies. The aim of this chapter is to introduce the readers to the concept of TMAs and discuss the existing and potential applications of TMA technology in toxicological immunohistochemistry. Despite its impact on other areas of research, TMA has not yet gained recognition in the area of toxicology. TMA technology is likely to have an increasingly important role in toxicology research and it is hoped that this chapter is to bring toxicologists up to date with the basic principles and future capabilities of TMAs.
What Are TMAs?
TMAs are an ordered array of tissue cores on a glass slide. They permit immunohistochemical analysis of numerous tissue sections under identical experimental conditions. The arrays can contain samples of every organ in the human body, or a wide variety of common tumors and obscure clinical cases alongside normal controls. The arrays can also contain pellets of cultured tumor cell lines. These arrays may be used like any histological section for immunohistochemistry and in situ hybridization to detect protein and gene expression.
The TMA technique was originally described in 1987 by Wan, Fortuna, and Furmanski in the Journal of Immunological Methods [16]. They published a modification of Battifora’s “sausage” block technique whereby tissue cores were placed in specific spatially fixed positions in a block [17]. The technique was popularized by Kononen and colleagues in the laboratory of Ollie Kallioneimi after they published a paper in Nature Medicine in 1998 [15].
This technology will allow investigators to analyze numerous biomarkers over essentially identical samples, develop novel prognostic markers, and validate potential drug targets. The ability to combine TMA technology with DNA microarrays and proteomics makes it a very attractive tool for the analysis of gene expression in clinically stratified tumor specimens and to relate the expression of each particular protein with clinical outcome. Public domain software allows researchers to examine digital images of individual histological specimens from TMAs, evaluate and score them, and store the quantitative data in a relational database. TMA technology may be specifically applied to the profiling of proteins and biomarkers of interest in other pathophysiological conditions such as congestive heart failure, renal disease, hypertension, diabetes, cystic fibrosis, and neurodegenerative disorders. This technology has the capacity to allow quantitative assessment of changes in protein markers across a range of tissues samples from multicenter toxicological studies. However, thus far, very few toxicologists have taken advantage of this technology.
Why Use TMAs?
A classic problem in biology is validation of a new concept on actual human tissue. A frequent problem is there is not enough tissue, either in amount or case number, to complete the analysis. This chronic shortage of tissue is partly due to the fact that the initial sampling is typically done by a pathologist whose primary goal is to provide a diagnostic consultation, not material for future experimentation. The difficulty of working with human tissue is further complicated by increasingly stringent guidelines regarding obtaining informed consent for its use. Thus, when tissue is obtained, it should be optimally managed to maximize its value. Tissue microarray represents a mechanism for highly effective use of this scarce resource.
In terms of benefits TMA technology offers numerous advantages including amplification of a scarce resource (i.e., rare archival tissue specimens; monoclonal or polyclonal antibodies that may no longer be available), experimental uniformity, decreased assay volume and use of antibodies, and significantly reduced or eliminated slide-to-slide variation.
How Is TMAs Produced?
TMAs are produced by re-locating tissue from conventional histologic paraffin blocks such that tissue from multiple patients or blocks can be seen on the same slide when the TMA paraffin block is sectioned. This is done by using a needle to biopsy a standard histologic section and placing the core into an array on a recipient paraffin block .
A standard histologic section is about 3–5 mm thick, with variation depending on the submitting pathologist or tech. After use for primary diagnosis, the sections can be cut 50–100 times depending on the care and skill of the sectioning technician. Thus, on average, each archived block might yield material for a maximum of 100 assays. If this same block is processed for optimal microarray construction, it could routinely be needle biopsied 200–300 times or more depending on the size of the tumor in the original block (theoretically it could be biopsied 1000s of times based on calculations of area, but empirically, 200–300 is selected as a conservative estimation) Then, once tissue microarrays are constructed, they can be judiciously sectioned in order to maximize the number of sections cut from an array. The sectioning process uses a tape-based sectioning aid (from Instrumedics Inc.6) that allows cutting of thinner sections. Optimal sectioning of arrays is obtained with about 2–3 μm sections. Thus, instead of 50–100 conventional sections or samples for analysis from one tissue biopsy, the microarray technique could produce material for 500,000 assays (assuming 250 biopsies per section times 2000 2.5 μm sections per 5 mm array block) represented as 0.6 mm disks of tissue. Thus, this technique essentially amplifies (up to 10,000-fold) the limited tissue resource.
Using this technology, each tissue sample is treated in an identical manner. Like conventional formalin-fixed paraffin-embedded material, tissue microarrays are amenable to a wide range of techniques including histochemical stains, immunologic stains with either chromogenic or fluorescent visualization, in situ hybridization (including both mRNA ISH and FISH), and even tissue microdissection techniques. For each of these protocols, conventional sections can have substantial slide-to-slide variability associated with processing 300 slides (e.g., 20 batches of 15 slides). The tissue microarrays allow the entire cohort to be analyzed in one batch on a single slide. Thus, reagent concentrations are identical for each case, as are incubation times and temperatures, wash conditions, and antigen retrieval, if necessary. Another significant advantage is that only a very small (a few μl) amount of antibody reagent is required to analyze an entire cohort. This advantage raises the possibility of use of tissue microarrays in screening procedures (e.g., in hybridoma screening), a protocol that is impossible using conventional sections. It also saves money when reagents are costly. Finally, there are occasions where the original block must be returned to the donating institution. In these cases, the block may be cored a few times without destroying the block. Then upon subsequent sectioning, it is still possible to make a diagnosis, even though tissue has been taken for array-based studies.
TMA Resources
In this section, TMAs and TMA services of publicly funded noncommercial and commercial sources are briefly summarized and discussed. Although the author has used commercial TMAs in one of his research papers [18], he does not have any affiliations with commercial TMA manufacturers. In most of the publications arising from our research laboratory, we have used noncommercial TMAs. We have also used custom-made low-density TMAs in studies of aquaporin water channel expression across kidneys of several mammalian species [18–22].
CHTN TMAs
The Cancer Diagnosis Program of the National Cancer Institute (NCI7) initiated the Cooperative Human Tissue Network (CHTN8 , 9) in 1987 to provide increased access to human tissue for basic and applied scientists from academia and industry to accelerate the advancement of discoveries in cancer diagnosis and treatment. The CHTN is a unique resource that provides remnant human tissues and fluids from routine procedures to investigators who utilize human biospecimens in their research. The CHTN comprises five adult divisions and one pediatric division. Each of the adult divisions coordinates investigator applications/requests based upon the investigator’s geographic location within North America. The Pediatric Division manages all investigators who request pediatric specimens only. The CHTN divisions share coordination for requests from outside North America.
Unlike tissue banks, the CHTN works prospectively with each investigator to tailor specimen acquisition and processing to meet their specific project requirements. Because the CHTN is funded by the NCI, the CHTN is able to maintain nominal processing fees for its services. The purpose of CHTN is to provide biomedical researchers access to human tissue specimens by offering tissue microarrays (TMAs) of neoplastic and non-neoplastic human tissues (see Fig. 2.4). The CHTN divisions work both independently with individual investigators and together as a seamless unit to fulfill requests that are difficult to serve by any single division. The CHTN’s unique informatics system allows each division to effectively communicate and network the needs of its investigators to all CHTN divisions. The Network as a whole can then help fulfill an investigator’s specific request.
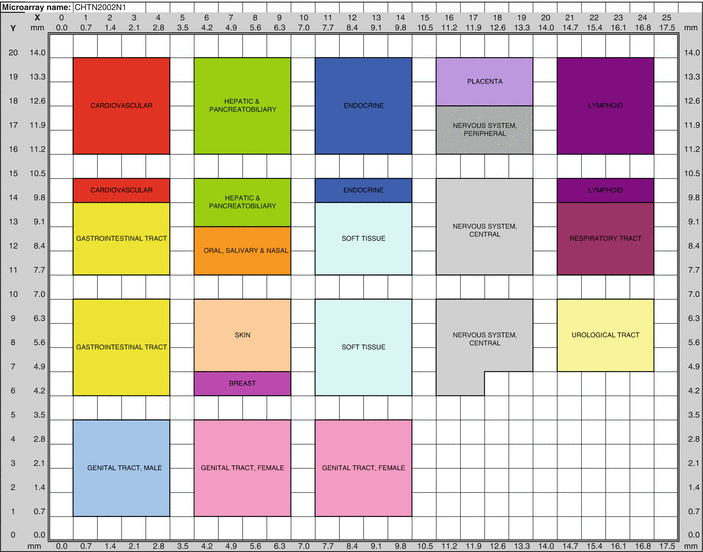

Fig. 2.4




Tissue microarray of formalin-fixed paraffin-embedded samples that includes 66 normal tissue types present in the human body. This microarray which is available from the Cooperative Human Tissue Network (CHTN) is codenamed CHTN2002N1. For a list of tissues corresponding to the codes, see the CHTN website (http://www.chtn.nci.nih.gov/)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


