CLASSIFICATION
A histologic classification of thyroid tumors is shown in
Table 7.1. It incorporates main principles of the current World Health Organization (WHO) classification of thyroid tumors.
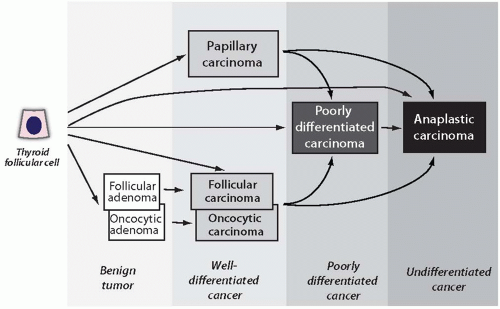
1 Thyroid tumors can be broadly subdivided into primary and secondary or metastatic tumors. Metastatic thyroid tumors are rare. Most primary thyroid tumors are epithelial tumors that originate from thyroid follicular cells. This group encompasses the most common types of thyroid neoplasms, that is, benign follicular adenoma and malignant well-differentiated papillary and follicular carcinomas. Poorly differentiated and anaplastic carcinomas also originate from follicular cells, and many of them are believed to develop as a result of dedifferentiation of a well-differentiated papillary or follicular carcinoma (
Fig. 7.1). It is likely that some follicular carcinomas arise as a result of malignant transformation of preexisting follicular adenomas, whereas others may bypass this premalignant stage. Medullary carcinomas originate from thyroid parafollicular or C cells.
Current thyroid tumor classifications encounter several controversial issues. One is related to the placement of thyroid oncocytic (Hürthle cell) adenomas and carcinomas. These tumors display several basic histologic features that are similar to those of conventional follicular tumors (i.e., follicular growth pattern, encapsulation) but are different in other microscopic features, in certain clinical characteristics, and possibly in some molecular alterations. Oncocytic adenomas and carcinomas are considered by some as separate types of thyroid tumors and by others as a variant of follicular adenoma and follicular carcinoma. The current WHO classification designates them as a variant of follicular tumors.
1 This may change in the future as new molecular studies characterizing these tumors emerge.
Poorly differentiated thyroid carcinoma is a distinct type of thyroid cancer. However, the diagnostic criteria for this tumor have not been universally accepted. On the basis of the characteristic growth pattern, many of these tumors are being designated as insular carcinomas. This term, however, places emphasis on the growth pattern rather than the cytologic features of tumor cells, and its use should be discouraged. The consensus diagnostic criteria for poorly differentiated carcinoma have been proposed based on the results of an international conference held in Turin, Italy in 2006.
2 When diagnosed based on these criteria, this tumor falls into a distinctly intermediate prognostic category between indolent well-differentiated papillary and follicular carcinomas and almost always lethal anaplastic carcinoma.
The placement of hyalinizing trabecular tumor represents another problematic issue for the classification of thyroid tumors. This rare tumor had been originally described as hyalinizing trabecular adenoma and typically has a benign course, despite sharing several histologic features with papillary carcinoma.
3 More recent studies suggest that this tumor may harbor molecular alterations characteristic of papillary carcinomas.
4,
5 Although, currently, there is no sufficient evidence to reclassify this tumor as a variant of papillary carcinoma, it deserves placement into a separate classification group.
Owing to the difficulty in diagnosing the encapsulated follicular variant of papillary carcinoma and the uncertainty in the malignant behavior of tumors with only partially developed nuclear features of papillary carcinoma, attempts have been made to introduce a different terminology for these tumors, such as “well-differentiated tumor of uncertain malignant potential.”
6 This terminology, however, is not widely accepted, in part because of the lack of information on the implication of this diagnosis for clinical management of these patients.
INCIDENCE AND EPIDEMIOLOGY
Thyroid cancer is the most common type of endocrine malignancy. It constitutes 3% of all newly diagnosed cancer cases in the United States
7 and 1.7% of all newly diagnosed cancer cases worldwide.
8 The incidence of thyroid cancer is about three times higher among females than males in the United States and most of other countries.
7,
8 For example, in the United States, the age-adjusted incidence of thyroid cancer in 2008 was 6.47 per 100,000 men and 19.39 per 100,000 women based on the Surveillance, Epidemiology, and End Results (SEER) national cancer data registry.
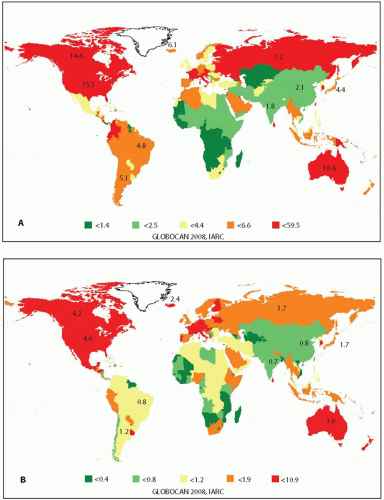
9 Worldwide, the incidence varies in different geographic regions and is overall higher in more economically developed countries. The highest rates are observed in South Korea, Northern America, Australia, and the several countries of Europe and Middle East (
Fig. 7.2).
8 The incidence varies between different ethnic groups. In the United States, thyroid cancer is about twice more common in whites than in blacks.
10,
11Thyroid cancer is rare in children, but its incidence begins to rise sharply in the second decade of life and peaks during the fifth and sixth decades of life (
Fig. 7.3A). This age distribution reflects the incidence of the three most common types of thyroid cancer, namely, papillary carcinoma that constitutes ˜80% of all thyroid cancer cases, follicular carcinoma (15%), and medullary carcinoma (3%).
10,
12 Anaplastic carcinoma, which accounts for <2% of thyroid tumors, typically occurs in the older age group and its incidence continues to rise with age.
The incidence of thyroid cancer has been steadily rising over the past three decades, and particularly since the mid-1990s, in many countries around the world.
13,
14,
15 On the basis of the SEER data, the incidence in the United States practically tripled since 1973, growing at a rate of 2.4% per year in 1980 to 1997 and 6.5% per year since 1997 (
Fig. 7.3B).
9 In fact, thyroid cancer is currently the fastest-increasing cancer in the United States in both men and women and has become the fifth most common type of cancer in women.
7 Mortality from thyroid cancer remains low (
Fig. 7.3B), although it has been rising since 1992 at a rate of 0.6% per year.
7The increase in incidence is almost entirely attributed to papillary carcinoma, whereas the rates of follicular, medullary, and
anaplastic cancer did not change significantly (
Fig. 7.4).
14,
16,
17 The increase affects both classic papillary carcinoma and the follicular variant of papillary carcinoma and is more pronounced for tumors of 1 cm or less in size.
18 However, the incidence of tumors >1 cm and even >4 to 5 cm in size is also on the rise.
17,
19,
20 Although exact reasons for the rising incidence of thyroid cancer are not entirely clear, several factors are likely to play a role. First, it may be in part because of improved cancer detection by ultrasonography and thyroid fine needle aspiration (FNA).
21,
22 Thyroid ultrasound has been used increasingly since the 1980s and can detect thyroid nodules as small as 0.2 cm in size. Under ultrasound guidance, even very small nodules can be biopsied using FNA technique and examined cytologically. These advances in health care are likely to have resulted in the increased detection of small asymptomatic cancer nodules, which are highly prevalent in the general population and rarely progress to a clinically relevant disease.
23 Second, the increase may reflect a better recognition of the follicular variant of papillary carcinoma. In the past, many of the tumors currently diagnosed as the follicular variant of papillary carcinoma were interpreted as follicular carcinomas. In addition, owing to a progressive decrease in the stringency of microscopic criteria for the diagnosis of the follicular variant of papillary carcinoma during the 1990s, tumors with partially developed nuclear features of papillary carcinoma, which had been diagnosed as benign lesions in the past, are now more likely to be interpreted as malignant.
24 However, it is unlikely that these factors are responsible for the entire increase in thyroid cancer incidence. In fact, recent studies suggest that the proportion of papillary carcinoma carrying a
BRAF mutation, which is a marker of more aggressive cancer, has been constant or even increasing during the last decades.
25,
26 The growing incidence of
BRAF-positive tumors and tumors >1 cm argues against
the possibility that thyroid cancer increase is solely because of better medical surveillance and detection of small, clinically irrelevant, nonprogressive thyroid cancers.
GENETIC SUSCEPTIBILITY AND OTHER RISK FACTORS
The development of thyroid tumors is likely to involve the interaction between the genetic predisposition, the endogenous hormonal factors, and the environmental risk factors (
Table 7.2). The role of genetic predisposition is best established for medullary carcinoma. Approximately 25% of these tumors occur as part of one of the multiple endocrine neoplasia (MEN) syndromes, MEN type 2A, MEN type 2B, and familial medullary thyroid carcinoma (FMTC). The disease is caused by a germline mutation in the
RET gene and has an autosomal dominant inheritance with almost complete penetrance but variable expressivity.
Approximately 5% of thyroid cancers of follicular cell origin show familial occurrence. Those are either associated with well-defined hereditary cancer syndromes or inherited through yet unidentified genetic mechanisms.
27 Overall, the risk of thyroid cancer in the first-degree relatives of patients with follicular cell-derived thyroid cancer is 4- to 10-folds higher than that in the general population.
28,
29 One of the known genetic syndromes is familial adenomatous polyposis (FAP), which is caused by a germline mutation of the adenomatous polyposis coli (
APC) gene on 5q21. Thyroid tumors in these patients typically manifest during the third decade of life and affect predominantly females.
30 Most of the FAP-associated thyroid tumors are papillary carcinomas, and they exhibit distinct histologic features.
31 Thyroid tumors are found in patients affected by
PTEN-hamartoma tumor syndrome, which encompass Cowden syndrome and other rare syndromes caused by a germline mutation of the
PTEN gene on 10q23.31. Most of these tumors are follicular carcinomas, although papillary carcinomas may also be seen in these patients.
32 Thyroid neoplasms may also present as a rare manifestation of the Carney complex, Werner syndrome, Peutz-Jeghers syndrome, and MEN syndromes. Most familial follicular cell-derived thyroid cancers, however, are not part of the known inherited syndromes. These tumors are also known as familial nonmedullary thyroid cancer. Most of these tumors (˜90%) are papillary carcinomas, and they are presumed to have an autosomal dominant inheritance with reduced penetrance.
33,
34 The inherited nature of these tumors is established with certainty when three or more first-degree relatives are diagnosed with thyroid cancer.
35,
36 Genetic linkage studies have mapped susceptibility loci to chromosomes 19p13.2, 1q21, and 2q21, although no specific susceptibility genes have been identified in these regions yet.
33Exposure to ionizing radiation during childhood is a well-established risk factor for thyroid cancer. Both external radiation (X-ray and γ-radiation) and internal exposure to radioiodine
131I result in the increased risk, although the risk from exposure to radioiodine
131I is clearly established only after accidental exposure during childhood. The risk has strong inverse correlation with age at exposure and is linear for thyroid doses in the range of 0.1 to 2 Gy received by children <15 years old.
37,
38 The minimal latent period for thyroid cancer development is 4 years after exposure, and the risk remains elevated for >40 years.
39,
40,
41 Most radiation-induced cancers are papillary
carcinomas, many of which show a solid growth pattern and are diagnosed as the solid variant of papillary carcinoma.
42 The risk of developing follicular cancer and benign nodules after radiation exposure is overall less pronounced, and these lesions may develop after a longer latency. Molecular mechanisms of radiation-associated papillary carcinogenesis primarily involve the generation of chromosomal rearrangements, such as
RET/PTC, whereas point mutations of
BRAF and other genes are much less common in these tumors.
43,
44,
45 The most recent outbreak of radiation-induced thyroid cancer has been registered in the populations of children and young adults exposed to radiation after the Chernobyl nuclear accident. In other regions of the world, therapeutic radiation for cancers of other locations is the leading source of exposure. In the United States, a history of prior exposure to radiation is found in ˜5% of patients with thyroid cancer.
12 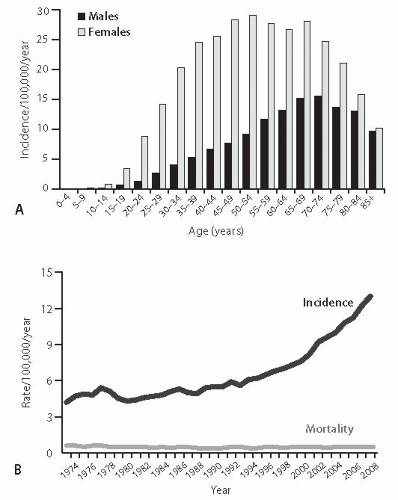


 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access