THE THYROID GLAND
The main anlage of the thyroid gland develops as a median endodermal downgrowth from the first and second pharyngeal pouches (Figure 16–1). During its migration caudally, it contacts the ultimobranchial bodies developing from the fourth pharyngeal pouches. When it reaches the position it occupies in the adult, with the isthmus situated just below the cricoid cartilage, the thyroid divides into two lobes. The site from which it originated persists as the foramen cecum at the base of the tongue. The path the gland follows may result in thyroglossal remnants (cysts) or ectopic thyroid tissue (lingual thyroid). A pyramidal lobe is frequently present. Agenesis of one thyroid lobe, almost always the left, may occur.
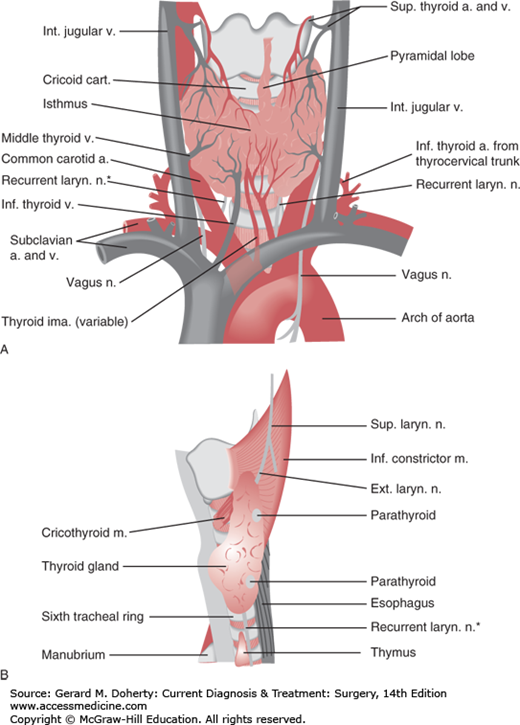
The normal thyroid weighs 15-25 g and is attached to the trachea by loose connective tissue. It is a highly vascularized organ that derives its blood supply principally from the superior and inferior thyroid arteries. A thyroid ima artery may also be present.
The function of the thyroid gland is to synthesize, store, and secrete the hormones thyroxine (T4) and triiodothyronine (T3). Iodide is absorbed from the gastrointestinal tract and actively trapped by the acinar cells of the thyroid gland. It is then oxidized and combined with tyrosine in thyroglobulin to form monoiodotyrosine (MIT) and diiodotyrosine (DIT). These are coupled to form the active hormones T4 and T3, which initially are stored in the colloid of the gland. Following hydrolysis of the thyroglobulin, T4 and T3 are secreted into the plasma, becoming almost instantaneously bound to plasma proteins. Most T3 in euthyroid individuals, however, is produced by extrathyroidal conversion of T4 to T3.
The function of the thyroid gland is regulated by a feedback mechanism that involves the hypothalamus and pituitary. Thyrotropin-releasing factor (TRF), a tripeptide amide, is formed in the hypothalamus and stimulates the release of the thyroid-stimulating hormone (TSH) thyrotropin, a glycoprotein, from the pituitary. Thyrotropin binds to TSH receptors on the thyroid plasma membrane, stimulating increased adenylyl cyclase activity; this increases cyclic adenosine monophosphate (cAMP) production and thyroid cellular function. Thyrotropin also stimulates the phosphoinositide pathway and—along with cAMP—stimulates thyroid growth.
In a patient with enlargement of the thyroid (goiter), the history (including local and systemic systems and family history) and examination of the gland are most important and are complemented by the selective use of thyroid function tests. The surgeon must develop a systematic method of palpating the gland to determine its size, contour, consistency, nodularity, and fixation and to examine for displacement of the trachea and the presence of palpable cervical lymph nodes. Normal or abnormal structures that are attached to the larynx, such as the thyroid gland, move cephalad with deglutition, whereas adjacent lymph nodes typically do not. The isthmus of the thyroid gland crosses the front of the trachea immediately caudal to the cricoid cartilage.
Thyroid function is assessed by highly sensitive TSH assays that can differentiate among patients with hypothyroidism (increased TSH levels), euthyroidism, and hyperthyroidism (decreased TSH levels). In most cases, therefore, serum T3, T4, and other variables need not be measured. A free T4 level is helpful in monitoring patients during treatment for Graves disease because the TSH level may remain suppressed despite the patient’s status improving. A serum T3 level is useful for diagnosing T3 toxicosis (high T3 and low TSH), or the euthyroid sick, low T3 syndrome (low T3 and normal or slightly increased TSH).
Radioactive iodine (RAI) uptake is useful for differentiating between hyperthyroidism and increased secretion of thyroid hormone (low TSH and increased RAI uptake) on the one hand and subacute thyroiditis (low TSH and low RAI uptake) on the other. Patients with the latter “leak” thyroid hormone from the gland, which suppresses serum TSH levels and, consequently, iodine uptake by the thyroid. Patients with Graves disease have increased levels of thyroid-stimulating immunoglobulins that increase iodine uptake despite low TSH levels.
DISEASES OF THE THYROID
ESSENTIALS OF DIAGNOSIS
Nervousness, weight loss with increased appetite, heat intolerance, increased sweating, muscular weakness and fatigue, increased bowel frequency, polyuria, menstrual irregularities, infertility
Goiter, tachycardia, atrial fibrillation, warm moist skin, thyroid thrill and bruit, cardiac flow murmur; gynecomastia
Eye signs: stare, lid lag, exophthalmos
TSH low or absent; TSI, iodine uptake, T3 and T4 increased; T3 suppression test abnormal (failure to suppress radioiodine uptake)
Hyperthyroidism is caused by the increased secretion of thyroid hormone (Graves disease, Plummer disease, iodine-induced [jodbasedow effect], amiodarone toxicity, TSH-secreting pituitary tumors, human chorionic gonadotropin [hCG]-secreting tumors), or by other disorders that increase thyroid hormone levels without increasing thyroid gland secretion (excess exogenous thyroid hormone intake, subacute thyroiditis, struma ovarii, and, rarely, metastatic thyroid cancers that secrete excess thyroid hormone). The most common causes of hyperthyroidism are diffusely hypersecretory goiter (Graves disease) and nodular toxic goiter (Plummer disease).
In all forms, the symptoms of hyperthyroidism are due to increased levels of thyroid hormone in the blood stream. The clinical manifestations of thyrotoxicosis may be subtle or marked and tend to go through periods of exacerbation and remission. Some patients ultimately develop hypothyroidism spontaneously (~ 15%) or as a result of treatment. Graves disease is an autoimmune disease—often with a familial predisposition—whereas the etiology of Plummer disease is unknown. Most cases of hyperthyroidism are easily diagnosed on the basis of the signs and symptoms; others (eg, mild or apathetic hyperthyroidism—which occurs most commonly in the elderly) may be recognized only with laboratory testing for a suppressed TSH level.
Thyrotoxicosis has been described with a normal T4 concentration, normal or elevated radioiodine uptake, and normal protein binding but with increased serum T3 by RIA (T3 toxicosis). T4 pseudothyrotoxicosis is occasionally seen in critically ill patients and is characterized by increased levels of T4 and decreased levels of T3 due to failure to convert T4 to T3. Thyrotoxicosis associated with toxic nodular goiter is usually less severe than that associated with Graves disease and is only rarely if ever associated with the extrathyroidal manifestations of Graves disease such as exophthalmos, pretibial myxedema, thyroid acropathy, or periodic hypocalcemic paralysis.
If left untreated, thyrotoxicosis causes progressive and profound catabolic disturbances and cardiac damage. Death may occur in thyroid storm or because of heart failure or severe cachexia.
The clinical findings are those of hyperthyroidism as well as those related to the underlying cause (Table 16–1). Nervousness, increased diaphoresis, heat intolerance, tachycardia, palpitations, fatigue, and weight loss in association with a nodular, multinodular, or diffuse goiter are the classic findings in hyperthyroidism. The patient may have a flushed and staring appearance. The skin is warm, thin, and moist, and the hair is fine.
| Clinical Manifestations | Frequency |
|---|---|
| Tachycardia | Nearly all |
| Nervousness | Nearly all |
| Goiter | Nearly all |
| Skin changes | Nearly all |
| Tremor | Nearly all |
| Increased sweating | Majority |
| Hypersensitivity to heat | Majority |
| Palpitations | Majority |
| Fatigue | Majority |
| Weight loss | Majority |
| Bruit over thyroid | Majority |
| Dyspnea | Majority |
| Eye signs | Majority |
| Weakness | Majority |
| Increased appetite | Majority |
| Eye complaints | Majority |
| Leg swelling | Some |
| Hyperdefecation (without diarrhea) | Some |
| Diarrhea | Some |
| Atrial fibrillation | Some |
| Splenomegaly | Few |
| Gynecomastia | Few |
| Anorexia | Few |
| Liver palms | Few |
| Constipation | Few |
| Weight gain | Few |
In Graves disease, there may be exophthalmos, pretibial myxedema, or vitiligo, virtually never seen in single or multinodular toxic goiter. The Achilles reflex time is shortened in hyperthyroidism and prolonged in hypothyroidism. The patient on the verge of thyroid storm has accentuated symptoms and signs of thyrotoxicosis, with hyperpyrexia, tachycardia, cardiac failure, neuromuscular excitation, delirium, or jaundice.
Laboratory tests reveal a suppressed TSH and an elevation of T3, free T4, and radioactive iodine. A history of medications is important, since certain drugs and organic iodinated compounds affect some thyroid function tests, and iodide excess may result in either iodide-induced hypothyroidism or iodine-induced hyperthyroidism (jodbasedow effect). In mild forms of hyperthyroidism, the usual diagnostic laboratory tests are likely to be only slightly abnormal. In these difficult-to-diagnose cases, two additional tests are helpful: the T3 suppression test and the thyrotropin-releasing hormone (TRH) test. In the T3 suppression test, hyperthyroid patients fail to suppress the thyroidal uptake of radioiodine when given exogenous T3. In the TRH test, serum TSH levels fail to rise in response to administration of TRH in hyperthyroid patients.
Other findings include a high thyroid-stimulating immunoglobulin (TSI) level, low serum cholesterol, lymphocytosis, and occasionally hypercalcemia, hypercalciuria, or glycosuria.
Anxiety neurosis, heart disease, anemia, gastrointestinal disease, cirrhosis, tuberculosis, myasthenia and other muscular disorders, menopausal syndrome, pheochromocytoma, primary ophthalmopathy, and thyrotoxicosis factitia may be clinically difficult to differentiate from hyperthyroidism. Differentiation is especially difficult when the thyrotoxic patient presents with minimal or no thyroid enlargement. Patients may also have painless or spontaneously resolving thyroiditis and are hyperthyroid because of increased release of thyroid hormone from the thyroid gland. This condition, however, is self-limited, and treatment with antithyroid drugs, radioactive iodine, or surgery is rarely necessary.
Anxiety neurosis is perhaps the condition most frequently confused with hyperthyroidism. Anxiety is characterized by persistent fatigue usually unrelieved by rest, clammy palms, a normal sleeping pulse rate, and normal laboratory tests of thyroid function. The fatigue of hyperthyroidism is often relieved by rest, the palms are warm and moist, tachycardia persists during sleep, and thyroid function tests are abnormal.
Organic disease of nonthyroidal origin that may be confused with hyperthyroidism must be differentiated largely on the basis of evidence of specific organ system involvement and normal thyroid function tests.
Other causes of exophthalmos (eg, orbital tumors) or ophthalmoplegia (eg, myasthenia) must be ruled out by ophthalmologic, ultrasonographic, computed tomography (CT) or magnetic resonance imaging (MRI) scans, and neurologic examinations.
Hyperthyroidism may be effectively treated by antithyroid drugs, radioactive iodine, or thyroidectomy. Treatment must be individualized and depends on the patient’s age and general state of health, the size of the goiter, the underlying pathologic process, and the patient’s ability to obtain follow-up care.
The principal antithyroid drug used in the United States is methimazole, 30-100 mg orally daily; propylthiouracil (PTU), 300-1000 mg orally daily has become an infrequent choice due to side effects. These agents interfere with organic binding of iodine and prevent coupling of iodotyrosines in the thyroid gland. One advantage over thyroidectomy and radioiodine in the treatment of Graves disease is that drugs inhibit the function of the gland without destroying tissue; therefore, there is a lower incidence of subsequent hypothyroidism. This form of treatment is usually used in preparation for surgery or RAI treatment but may be used as definitive treatment. Reliable patients with small goiters are good candidates for this regimen. A prolonged remission after 18 months of treatment occurs in 30% of patients, some of whom eventually become hypothyroid. Side effects of PTU include rashes and fever (3%-4%), agranulocytosis (0.1%-0.4%), and, rarely, liver failure. Patients must be warned to immediately stop the drug, see a physician, and have a white blood cell count if sore throat or fever develops.
Radioiodine (131I) may be given safely after the patient has been treated with antithyroid medications and has become euthyroid. Radioiodine is indicated for patients who are over 40 years or are poor risks for surgery and for patients with recurrent hyperthyroidism. It is less expensive than operative treatment and is effective. Radioiodine treatment at doses necessary to treat hyperthyroidism does not appear to increase the risk of leukemia or of congenital anomalies. However, an increased incidence of benign thyroid tumors and, rarely, thyroid cancer has been noted to follow treatment of hyperthyroidism with radioiodine. In young patients, the radiation hazard is certainly increased, and the chance of developing hypothyroidism is virtually 100%. After the first year of treatment with radioiodine, the incidence of hypothyroidism increases about 3% per year.
Hyperthyroid children and pregnant women should not be treated with radioiodine.
The main advantages of thyroidectomy is a more rapid and certain control of the disease than can be achieved with radioiodine treatment. Surgery is often the preferred treatment: (1) in the presence of a very large goiter or a multinodular goiter with relatively low RAI uptake, (2) if there is a suspicious or malignant thyroid nodule, (3) for patients with ophthalmopathy, (4) for the treatment of pregnant patients or children, (5) for the treatment of women who wish to become pregnant within 1 year after treatment, and (6) for patients with amiodarone-induced hyperthyroidism.
The risk of thyroidectomy for toxic goiter is small since the introduction of the combined preoperative use of iodides and antithyroid drugs. Methimazole or another antithyroid drug is administered until the patient becomes euthyroid and is continued until the time of operation. Three drops of potassium iodide solution or Lugol iodine solution are then given for about 10 days before surgery in conjunction with the propylthiouracil as this may decrease the friability and vascularity of the thyroid, thereby technically facilitating thyroidectomy.
An occasional untreated or inadequately treated hyperthyroid patient may require an emergency operation for some unrelated problem such as acute appendicitis and thus require immediate control of the hyperthyroidism. Such a patient should be treated in a manner similar to one in thyroid storm, since thyroid storm or hyperthyroid crises may be precipitated by surgical stress or trauma. Treatment of hyperthyroid patients requiring an emergency operation or those in thyroid storm is as follows: prevent release of preformed thyroid hormone by administration of Lugol iodine solution or with ipodate sodium; give β-adrenergic blocking agents to antagonize the peripheral manifestations of thyrotoxicosis; and decrease thyroid hormone production and extrathyroidal conversion of T4 to T3 by giving propylthiouracil.
The combined use of β-blocking agents and iodide lowers serum thyroid hormone levels. Other important considerations are to treat precipitating causes (eg, infection, drug reactions); to support vital functions by giving oxygen, sedatives, intravenous fluids, and corticosteroids; and to reduce fever. Benzodiazepines may be useful in the patient in whom nervousness is a prominent symptom, and a cooling blanket should be used in patients if needed fir temperature control.
The treatment of hyperthyroidism by subtotal, near-total, or total thyroidectomy eliminates both the hyperthyroidism and the goiter. As a rule, nearly all of the thyroid gland is removed, sparing the parathyroid glands and the recurrent laryngeal nerves. A very complete total thyroidectomy is generally indicated for patients with Graves ophthalmopathy.
The death rate associated with these procedures is extremely low—less than 0.1%. Thyroidectomy thus provides safe and rapid correction of the thyrotoxic state. The frequency of recurrent hyperthyroidism and hypothyroidism depends on the amount of thyroid remaining and on the natural history of the hyperthyroidism. Given an accomplished surgeon and good preoperative preparation, injuries to the recurrent laryngeal nerves and parathyroid glands occur in less than 2% of cases. Adequate exposure and avoidance of injury to the recurrent laryngeal nerves and parathyroid glands are essential.
The pathogenesis of the ocular problems in Graves disease remains unclear. Evidence originally supporting the role of either long-acting thyroid stimulator (LATS) or exophthalmos-producing substance (EPS) has not been authenticated.
The eye complications of Graves disease may begin before there is any evidence of thyroid dysfunction or after the hyperthyroidism has been appropriately treated. Usually, however, the ocular manifestations develop concomitantly with the hyperthyroidism. Relief of the eye problems is often difficult to accomplish until coexisting hyperthyroidism or hypothyroidism is controlled.
The eye changes of Graves disease vary from no signs or symptoms to loss of sight. Mild cases are characterized by upper lid retraction and stare with or without lid lag or proptosis. These cases present only minor cosmetic problems and usually require no treatment unless the eyes are dry. When moderate to severe eye changes occur, there is retroorbital soft tissue involvement with proptosis, extraocular muscle involvement, and finally optic nerve involvement. Some cases may have marked chemosis, periorbital edema, conjunctivitis, keratitis, diplopia, ophthalmoplegia, and impaired vision. Ophthalmologic consultation is required.
Treatment of the ocular problems of Graves disease includes maintaining the patient in a euthyroid state without increase in TSH secretion, protecting the eyes from light and dust with dark glasses and eye shields, elevating the head of the bed, using diuretics to decrease periorbital and retrobulbar edema, and giving methylcellulose or guanethidine eye drops. High doses of glucocorticoids are beneficial in certain patients, but their effectiveness is variable and unpredictable. If exophthalmos progresses despite medical treatment, lateral tarsorrhaphy, retrobulbar irradiation, or surgical decompression of the orbit may be necessary. Total thyroidectomy is the treatment of choice when it can be done with a low risk of complications. Graves disease is more likely to worsen after radioiodine treatment than after thyroidectomy. It is important that patients with ophthalmopathy be made aware of the natural history of the disease and also that they be kept euthyroid, since hyperthyroidism and hypothyroidism may produce visual deterioration. Operations to correct diplopia should be deferred until after the ophthalmopathy has stabilized.
The clinician should determine whether a nodular goiter or thyroid nodule is causing localized or systemic symptoms and whether it is benign or malignant. The differential diagnosis includes benign goiter, intrathyroidal cysts, thyroiditis, benign and malignant tumors, and, rarely, metastatic tumors to the thyroid. The history should specifically emphasize the duration of swelling, recent growth, local symptoms (dysphagia, pain, or voice changes), and systemic symptoms (hyperthyroidism, hypothyroidism, or those from possible tumors metastatic to the thyroid). The patient’s age, gender, place of birth, family history, and history of radiation to the neck are most important. Low-dose therapeutic radiation (6.5-2000 cGy) in infancy or childhood is associated with an increased incidence of benign goiter (~ 35%) or thyroid cancer (~ 13%) in later life. A thyroid nodule is more likely to be a cancer in a man than in a woman, and in younger (under 20 years) and older (over 60 years) patients rather than in others. In certain geographic areas, endemic goiter and benign nodules are common. Thyroid cancer is familial in about 25% of patients with medullary thyroid cancer (familial medullary thyroid cancer, multiple endocrine neoplasia [MEN] types 2A and 2B) and in about 7% of patients with papillary or Hürthle cell cancer. Papillary thyroid cancer occurs more often in patients with Cowden syndrome, Gardner syndrome, or Carney syndrome.
The clinician must systematically palpate the thyroid to determine whether there is a solitary thyroid nodule or if it is a multinodular gland and whether there are palpable lymph nodes. A solitary hard thyroid nodule is likely to be malignant, whereas most multinodular goiters are benign. Ultrasound evaluation helps document the number of nodules, whether a nodule is suspicious for cancer, and whether there are coexistent suspicious lymph nodes.
In many patients, the possibility of cancer is difficult to exclude without microscopic examination of the gland itself. Percutaneous needle biopsy is the most cost-effective diagnostic test and, along with ultrasound, has replaced radioiodine scanning for the evaluation of nodules. Cytologic results are classified by the Bethesda criteria (Table 16–2). False-positive diagnoses of cancer are rare, but about 20% of biopsy specimens reported as one of the indeterminate categories (follicular lesion of unknown significance or neoplasms) and there are more rare falsely benign results. If the specimen is reported as inadequate, biopsy should be repeated. Needle biopsy is not as helpful in patients with a history of irradiation to the neck because radiation-induced tumors are often multifocal, and a negative biopsy may therefore be unreliable. About 40% of these patients will have thyroid cancer. Radioiodine scanning can be used selectively to determine whether a follicular neoplasm by cytologic examination is functioning (warm or hot) or nonfunctioning (cold). Hot solitary thyroid nodules may cause hyperthyroidism but are rarely malignant, whereas cold solitary thyroid nodules have an incidence of cancer of 15%-20%. Thyroid carcinoma is uncommon (~ 3%) in multinodular goiters, but if there is a dominant nodule or one that enlarges, it should be biopsied or removed. Thyroid cancer occurs in nearly 40% of the children with solitary thyroid nodules; therefore, fine-needle biopsy or thyroidectomy is indicated. Ultrasound differentiates solid and cystic lesions and, as mentioned, may detect enlarged lymph nodes. About 15% of cold solitary lesions are cystic. CT or MRI scans are usually not necessary but are helpful when the limits of the tumor cannot be defined, such as in patients with large, invasive, or substernal goiters or tumors.
| Cytology Result | Likelihood of Malignancy | Usual Management |
|---|---|---|
| Nondiagnostic | Unknown | Reaspiration |
| Benign | < 1% | Follow |
| Follicular lesion of undetermined significance | 5%-10% | Consider follow, reaspiration or molecular testing of aspirate |
| Follicular or Hürthle cell neoplasm | 20%-30% | Consider follow, reaspiration or molecular testing of aspirate |
| Suspicious for malignancy | 50%-75% | Generally treat as for malignancy |
| Malignant | 100% | Treat for malignancy |
The principal indications for surgical removal of a nodular goiter are: (1) suspicion of or documented cancer, (2) symptoms of pressure, (3) hyperthyroidism, (4) substernal extension, and (5) cosmetic deformity. Incidentally discovered thyroid nodules by ultrasonography, CT, MRI, or positron emission tomography (PET) scans should be evaluated by ultrasound, then, if indicated, fine-needle aspiration biopsy. Nonoperative treatment is indicated in patients with small or moderately sized multinodular goiters and Hashimoto thyroiditis unless there is a clinically suspicious area, a nodule that is growing, a personal history of radiation exposure or a family history of thyroid carcinoma.
Simple goiter may be physiologic, occurring during puberty or pregnancy, or it may occur in patients from endemic (iodine-poor) regions or as a result of prolonged exposure to goitrogenic foods or drugs. As the goiter persists, there is a tendency to form nodules. Goiter may also occur early in life as a consequence of a congenital defect in thyroid hormone production or in patients with Hashimoto thyroiditis. It is generally assumed that nontoxic goiter represents a compensatory response to inadequate thyroid hormone production, although thyroid growth immunoglobulins may also be important. Nontoxic diffuse goiter usually responds favorably to thyroid hormone administration.
Symptoms are usually awareness of a neck mass and dyspnea, dysphagia, or symptoms caused by interference with venous obstruction. In diffuse goiter, the thyroid is symmetrically enlarged and has a smooth surface; however, most patients have multinodular glands by the time they seek medical care. Thyroid function is usually normal, though the sensitive TSH may be suppressed and the radioiodine uptake increased. Surgery is indicated to relieve the pressure symptoms of a large goiter for substernal goiter or to rule out cancer when there are localized areas of hardness or rapid growth. Aspiration biopsy cytology is helpful in these patients.
The inflammatory diseases of the thyroid are termed acute, subacute, or chronic thyroiditis, which can be either suppurative or nonsuppurative.
Acute suppurative thyroiditis is uncommon and is characterized by the sudden onset of severe neck pain accompanied by dysphagia, fever, and chills. It usually follows an acute upper respiratory tract infection; can be diagnosed by percutaneous aspiration, smear, and culture; and is treated by surgical drainage. The organisms are most often streptococci, staphylococci, pneumococci, or coliforms. It may also be associated with a piriform sinus fistula. A barium swallow is therefore recommended in persistent or recurrent cases.
Subacute thyroiditis, a noninfectious disorder, is characterized by thyroid swelling, head and chest pain, fever, weakness, malaise, palpitations, and weight loss. Some patients with subacute thyroiditis have no pain (silent thyroiditis), in which case the condition must be distinguished from Graves disease. In subacute thyroiditis, the erythrocyte sedimentation rate and serum gamma globulin are almost always elevated, and radioiodine uptake is very low or absent with increased or normal thyroid hormone levels. The illness is usually self-limited, and aspirin and corticosteroids relieve symptoms. Most of these patients eventually become euthyroid.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree