Thyroid Function
It is important to understand thyroid biochemistry, as it helps to interpret thyroid function tests, which are among the most common endocrine requests in clinical practice. Thyroid disease is relatively common, and therefore is likely to be encountered clinically.
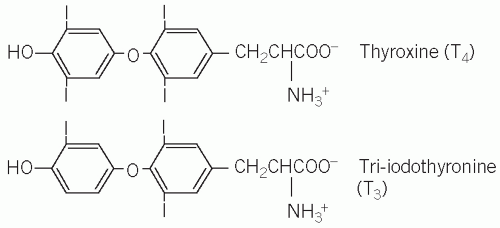
Briefly, thyroxine (T4), tri-iodothyronine (T3) and calcitonin are secreted by the thyroid gland. Both T4 and T3 are products of the follicular cells and influence the rate of all metabolic processes. Calcitonin is produced by the specialized C cells and influences calcium metabolism (see Chapter 6).
PHYSIOLOGY
Thyroid hormones are synthesized in the thyroid gland by the iodination and coupling of two molecules of the amino acid tyrosine, a process that is dependent on an adequate supply of iodide. Iodide in the diet is absorbed rapidly from the small intestine. In areas where the iodide content of the soil is very low, there used to be a high incidence of enlargement of the thyroid gland (goitre), but the general use of artificially iodized salt has made this a less common occurrence. Seafoods generally have a high iodide content. Therefore fish and iodized salt are the main dietary sources of the element. Normally about a third of dietary iodide is taken up by the thyroid gland and the rest is renally excreted.
Synthesis of thyroid hormones
Iodide is actively taken up by the thyroid gland under the control of thyroid-stimulating hormone (TSH) via a sodium/iodide symporter. Uptake is blocked by thiocyanate and perchlorate. The concentration of iodide in the gland is at least 20 times that in plasma and may exceed it by 100 times or more.
Iodide is rapidly converted to iodine within the thyroid gland, catalysed by thyroid peroxidase (TPO). Iodination of tyrosine residues in a large 660-kDa glycoprotein, thyroglobulin, takes place to form mono-iodotyrosine (MIT) and di-iodotyrosine (DIT) mediated by the enzyme TPO. This step is inhibited by carbimazole and propylthiouracil.
Iodotyrosines are coupled to form T4 (DIT and DIT) and T3 (DIT and MIT) (Fig. 11.1), which are stored in the lumen of the thyroid follicular cells. Normally much more T4 than T3 is synthesized, but, if there is an inadequate supply of iodide, the ratio of T3 to T4 in the gland increases. The thyroid hormones, still incorporated in thyroglobulin, are stored in the colloid of the thyroid follicle.
Prior to the secretion of thyroid hormones, thyroglobulin is taken up by the follicular cells, by a process involving endocytosis and then phagocytosis, and T4 and T3 are released by proteolytic enzymes into the bloodstream. This process is stimulated by TSH and inhibited by iodide. The thyroid hormones are immediately bound to plasma proteins. Monoiodotyrosine and DIT, released at the same time, are de-iodinated and the iodine is reused.
Each step is controlled by specific enzymes, and congenital deficiency of any of these enzymes can lead to goitre and, if severe, hypothyroidism. The uptake of iodide, as well as the synthesis and secretion of thyroid hormones, is regulated by TSH, secreted from the anterior pituitary gland. About 10 times more T4 than T3 is formed, with most of the latter being formed by de-iodination in the liver, kidneys and muscle.
Protein binding of thyroid hormones in plasma
Most of the plasma T4 and T3 is protein bound, mainly (70 per cent) to an α-globulin, thyroxine-binding globulin (TBG), and, to a lesser extent (15 per cent), transthyretin (previously called pre-albumin), with about 10-15 per cent bound to albumin. In keeping with many other hormones, the free unbound fraction is the physiologically active form, which also regulates TSH secretion from the anterior pituitary. Modern laboratory assays tend to measure the free hormones. Changes in the plasma concentrations of the binding proteins, particularly TBG, alter plasma total T4 and T3 concentrations, but not the concentrations of free hormones.
Peripheral conversion of thyroid hormone
Some of the circulating T4 is de-iodinated by enzymes in peripheral tissues, especially in the liver and kidneys. About 80 per cent of the plasma T3 is produced by the removal of an iodine atom from the outer (β) ring; the remaining 20 per cent is secreted by the thyroid gland. De-iodination of the inner (a) ring produces reverse T3, which is probably inactive. The T3 binds more avidly to thyroid receptors than T4 and is the main active form. The conversion of T4 to T3 may be:
reduced by many factors, of which the most important are:
systemic illness,
prolonged fasting,
drugs such as β-blockers, for example propranolol or amiodarone (200 mg of this anti-arrhythmic drug contains about 75 mg of iodine);
increased by drugs that induce hepatic enzyme activity, such as phenytoin.
The plasma T3 concentration is therefore a poor indicator of thyroid hormone secretion because it is influenced by many non-thyroidal factors and its measurement is rarely indicated, except if thyrotoxicosis is suspected.
Action of thyroid hormones
Thyroid hormones affect many metabolic processes, increasing oxygen consumption. They bind to specific receptors in cell nuclei and change the expression of certain genes. Thyroid hormones are essential for normal growth, mental development and sexual maturation and also increase the sensitivity of the cardiovascular and central nervous systems to catecholamines, thereby influencing cardiac output and heart rate.
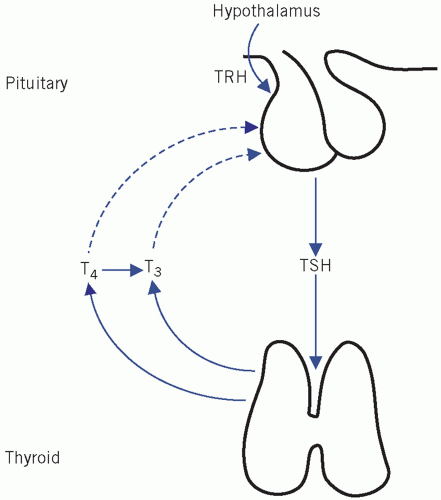
Control of thyroid-stimulating hormone secretion
Thyroid-stimulating hormone stimulates the synthesis and release of thyroid hormones from the thyroid gland. Its secretion from the anterior pituitary gland is controlled by thyrotrophin-releasing hormone (TRH) and circulating concentrations of thyroid hormones.
Effect of thyrotrophin-releasing hormone
Pituitary TSH synthesis and release are stimulated by TRH, a tripeptide produced in the hypothalamus and released into the portal capillary plexus. The action of TRH can be over-ridden by high circulating free T4 (fT4) concentrations, and therefore exogenous TRH has little effect on TSH secretion in hyperthyroidism (see later for TRH test). Once TRH reaches the pituitary, it binds to TRH receptors, members of the seven-transmembrane-spanning receptor family, which are coupled to G proteins.
Effects of thyroid hormones in the control of thyroidstimulating hormone secretion
Thyroid hormones reduce TSH secretion by negative feedback. Tri-iodothyronine binds to anterior pituitary nuclear receptors. In the anterior pituitary gland, most of the intracellular T3 is derived from circulating fT4. Therefore this gland is more sensitive to changes in plasma T4 than to T3 concentrations.
The secretion and control of thyroid hormones is summarized in Figure 11.2.
THYROID FUNCTION TESTS
Assessment of thyroid hormone secretion can be made by measuring plasma TSH as well as either fT4 or total T4 [sometimes also free T3 (fT3) or total T3]. Each test has its advantages and disadvantages, although probably most laboratories now offer fT4 and fT3 assays rather than total hormone concentrations. Thus, regarding assays this chapter will mainly discuss free hormones.
Plasma thyroid-stimulating hormone
Concentrations of TSH are high in primary hypothyroidism and low in secondary or pituitary hypothyroidism. In hyperthyroidism, high plasma T4 and T3 concentrations suppress TSH release from the pituitary, resulting in very low or undetectable plasma TSH concentrations. Plasma TSH assays are used as first-line assays for thyroid function assessment. Newgeneration assays have high sensitivity and have a detection limit for plasma TSH of less than 0.1 mU/L.
In normal individuals there is a log-linear relationship between plasma fT4 and TSH concentrations; that is to say, exponential increases in TSH concentration occur with small incremental changes in fT4 concentration.
In normal individuals there is a log-linear relationship between plasma fT4 and TSH concentrations; that is to say, exponential increases in TSH concentration occur with small incremental changes in fT4 concentration.
Plasma total thyroxine or free thyroxine assays
Plasma T4 is more than 99 per cent protein bound; therefore, plasma total T4 assays reflect the proteinbound rather than the free hormone fraction. Total T4 reflects fT4 concentrations, unless there are abnormalities of binding proteins.
In the euthyroid state, about a third of the binding sites on TBG are occupied by T4 and the remainder are unoccupied, irrespective of the concentration of the binding protein.
In hyperthyroidism, both plasma total and fT4 concentrations are increased and the number of unoccupied binding sites on TBG is decreased.
In hypothyroidism, the opposite of the above occurs.
An increase in plasma TBG concentration causes an increase in both bound T4 and unoccupied binding sites but no change in plasma fT4 concentrations. Such an increase may occur because of:
a high oestrogen concentration during pregnancy or in the newborn infant,
oestrogen therapy, for example certain oral contraceptives or hormone replacement therapy,
inherited TBG excess (rare).
A decrease in plasma TBG concentration decreases both bound T4 concentrations and unoccupied binding sites, but does not alter the plasma fT4 concentration. Such changes may occur because of:
severe illness, but this is usually temporary,
loss of low-molecular-weight proteins, usually in the urine, for example nephrotic syndrome,
androgens or danazol treatment,
inherited TBG deficiency (rare).
These changes might be misinterpreted as being diagnostic of hyperthyroidism or hypothyroidism respectively if only plasma total T4 was assayed and it is for this reason that fT4 concentrations are now generally preferred.
Plasma total or free tri-iodothyronine
Total T3 or fT3 concentrations may help in the diagnosis of hyperthyroidism but are not usually used routinely to diagnose hypothyroidism because normal plasma concentrations are very low. In hyperthyroidism, the increase in plasma T3 or fT3 concentrations is greater, and usually occurs earlier than that of T4 or fT4.
Occasionally in hyperthyroidism the plasma T3 or fT3 concentrations are elevated but not those of T4 or fT4 (T3 toxicosis). Like T4, T3 is bound to protein. It is usually preferable to measure the plasma concentration of fT3 rather than total T3, as the latter may be altered by changes in the plasma concentrations of TBG.
Thyrotrophin-releasing hormone test
The TRH test is used to confirm the diagnosis of secondary hypothyroidism, or occasionally to diagnose early primary hypothyroidism. Since the development of sensitive TSH assays, it is rarely used to diagnose hyperthyroidism, although it may have a place in the differential diagnosis of thyroid resistance syndrome or TSH-secreting pituitary tumours (TSHomas).
It is sometimes used as part of the combined pituitary stimulation test (see Chapter 7).
Allergic reactions may occur, and therefore resuscitation facilities should be available and the test should be carried out by experienced staff.
Procedure
A basal blood sample is taken.
200 µg of TRH is injected intravenously over about a minute.
Note that certain drugs, such as dopamine agonists and glucocorticoids, reduce the response, and oestrogens, metoclopramide and theophylline enhance it.
Interpretation
In normal subjects, plasma TSH concentration increases at 20 min by at least 2 mU/L and exceeds the upper limit of the reference range, with a small decline at 6 min.
An exaggerated response at 20 min and a slight fall at 60 min are suggestive of primary hypothyroidism.
A normal or exaggerated increment but delayed response, with plasma TSH concentrations higher at 60 min than at 2 min, suggests secondary hypothyroidism. If clinically indicated, pituitary and hypothalamic function should be investigated.
A flat response of TSH of less than 5 mU/L is compatible with primary hyperthyroidism, although this may also occur in some euthyroid patients with multinodular goitre.
Table 11.1 Drug effects on thyroid function tests | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree