Genetic Syndromes
Papillary carcinomas have been described in patients with familial adenomatous polyposis coli (FAP), Cowden syndrome, hereditary nonpolyposis colon cancer syndrome (HNPCC), Peutz-Jeghers syndrome, and ataxia telangiectasia (
117,
118).
FAP is caused by germline mutations of the adenomatous polyposis coli (
APC) gene. Thyroid carcinoma, mostly papillary carcinoma (>95% of cases), occurs in 1% to 2% of patients with FAP; all these patients show germline mutations of the
APC gene; however, somatic mutations of or loss of heterozygosity (LOH) for
APC gene are not found in thyroid tumors. Interestingly, a majority of these tumors do show activation of
RET/PTC1 in thyroid tumors, suggesting a possible association between
APC and
RET/PTC in the development of this particular subset of familial papillary carcinoma (
119).
Cowden syndrome is characterized by formation of hamartomas in several organs and a high risk of developing breast and thyroid cancer. The genetic locus for Cowden syndrome has been mapped to chromosome 10q23.3 and is also known as
PTEN, which is a protein tyrosine phosphatase and exerts its tumor suppressor effects by antagonizing protein tyrosine kinase activity. Interestingly,
PTEN mutation or gene deletion is noted in 26% of benign tumors but only in 6.1% of malignant tumors of the thyroid (
120,
121).
Pathology
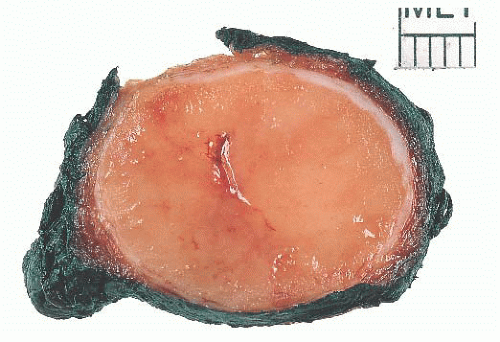
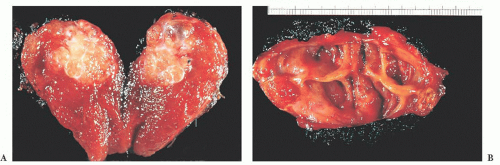
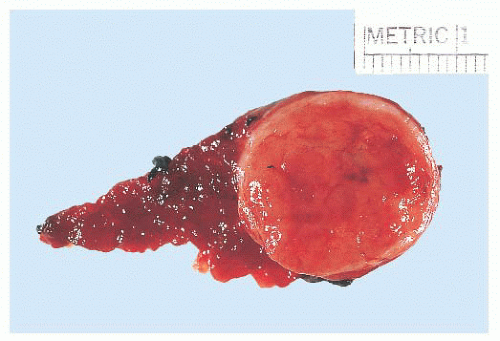
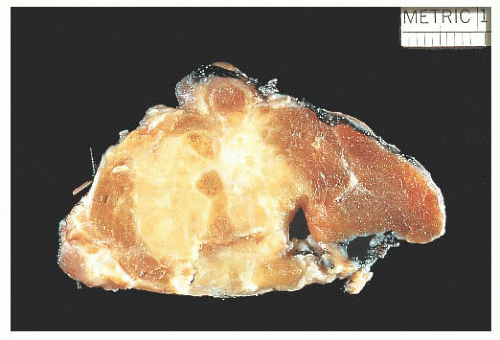
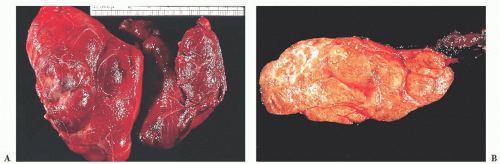
The gross appearance of PTC is quite variable (
71,
124). The lesions may appear anywhere within the gland. By definition, typical papillary carcinomas are greater than 1.0 to 1.5 cm, often averaging 2 to 3 cm, although lesions may be quite large (
Fig. 13.9). The lesions are firm and usually white in color with an invasive appearance. Lesional calcification is a common feature. Because of extensive sclerosis, the lesion may grossly resemble a scar. In addition, cyst formation may be observed. In fact, some lesions may rarely be completely cystic, making diagnosis difficult (
97). Necrosis (in the absence of a prior needle biopsy) is not a feature of typical papillary carcinoma and suggests a higher grade lesion (
125).
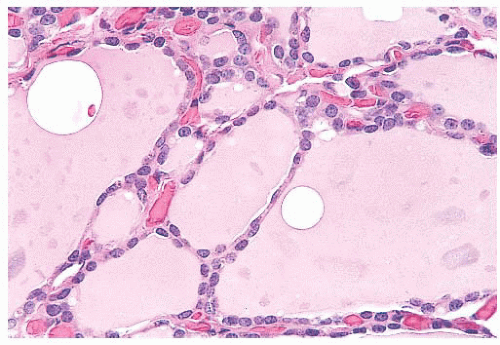
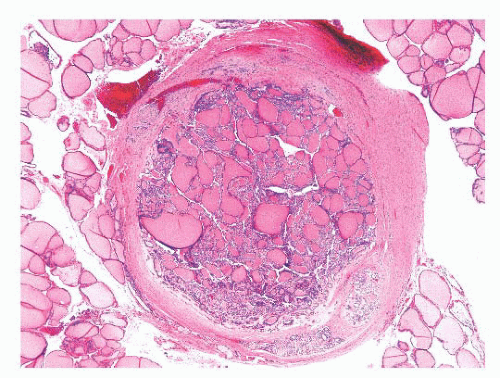
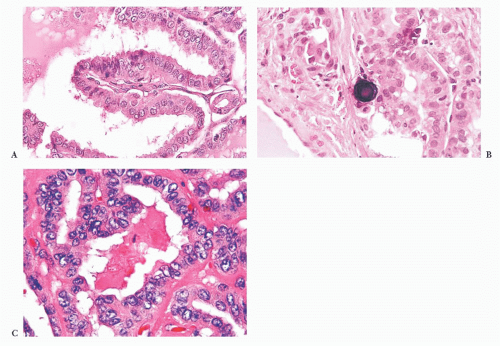
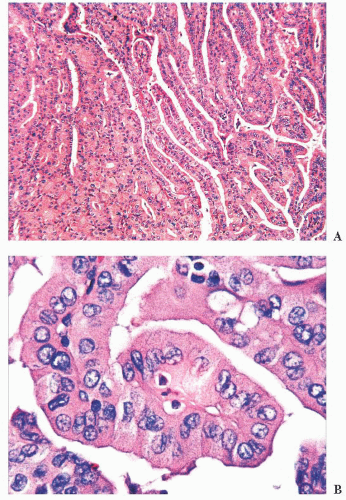
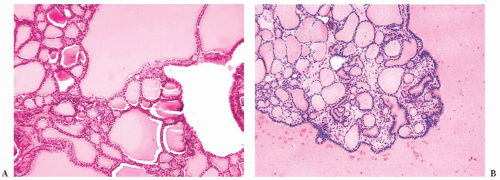
Microscopically, papillary carcinomas share certain features (
Table 13.2). The neoplastic papillae contain a central core
of fibrovascular (occasionally just fibrous) tissue lined by one or occasionally several layers of cells with crowded oval nuclei (
Fig. 13.10). In contrast, hyperplasia of thyroid follicles may sometimes exaggerate into papillary change; there is infolding of the lining epithelium composed of columnar cells with basal round and uniform nuclei. There is either no central core or a core of edematous or myxomatous paucicellular stroma often including small follicles (subfollicle formation) (
71,
110,
124).
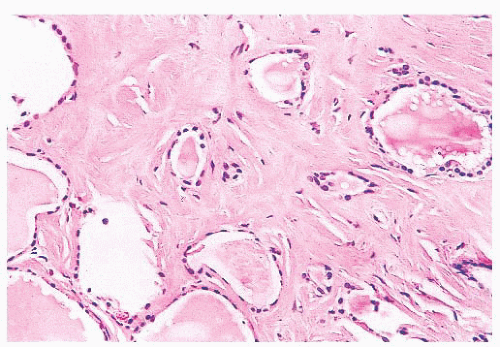
Psammoma bodies that represent the “ghosts” of dead papillae are differentiated from dystrophic calcifications by lamellations (
Fig. 13.10) (
126). True psammoma bodies are formed by focal areas of infarction of the tips of papillae attracting calcium that is deposited on the dying cells. Progressive infarction of the papillae and ensuing calcium deposition lead to lamellation (
127). Psammoma bodies are usually present within the cores of papillae or in the tumor stroma, but not within the neoplastic follicles. Only rarely are psammoma bodies found in benign conditions in the thyroid (
128).
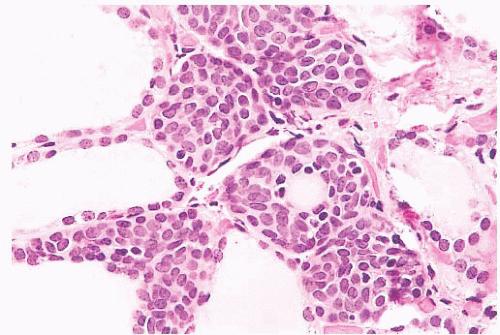
The nuclei of papillary cancer have been described as clear, ground glass, empty, or Orphan Annie-eyed (
Fig. 13.10). These nuclei are larger and more oval than normal follicular nuclei and contain hypodense chromatin (
71,
129). In papillary cancer, these nuclei often overlap one another. Although cleared nuclei are characteristic of papillary carcinoma, autoimmune thyroiditis, particularly Hashimoto disease, often shows similar nuclear changes (
42). Intranuclear inclusions of cytoplasm
are often found. Another characteristic of the papillary cancer nucleus is the nuclear groove (
97,
129). Nuclear grooves may be seen in other thyroid lesions including Hashimoto disease, adenomatous hyperplasia, and diffuse hyperplasia as well as in follicular adenomas (particularly hyalinizing trabecular neoplasm) (
130,
131). For the most part, nuclear grooves are more commonly seen in papillary carcinoma than in other thyroid lesions; however, the mere presence of nuclear grooves is not diagnostic for papillary carcinoma.
Most of these tumors will be composed predominantly or focally of papillary areas. A large number will contain follicular areas as well (
132). The tumor cells are usually columnar. Clear nuclei are found in more than 80% of such lesions, intranuclear inclusions in approximately 80% to 85%, and nuclear grooves are seen in almost all the cases. Mitoses are exceptional in usual papillary carcinoma. Psammoma bodies are found in approximately 40% to 50% of cases (
71), but their presence in thyroid tissue indicates that a papillary carcinoma is most likely present somewhere in the gland. The finding of psammoma bodies in a cervical lymph node is strong evidence of a papillary cancer in the thyroid (
14).
Many papillary carcinomas (approximately 15% to 45%) contain foci of squamous differentiation (
133). Almost all papillary carcinomas show areas of desmoplasia either in the central portions of the tumor or at the peripheral zones of the lesions. Even in encapsulated lesions, sclerosis is seen in areas of capsular penetration (
124).
Scattered lymphocytes are common at the invasive edges of the tumor (
124,
134). Rarely, an intense lymphocytic infiltrate is seen, but it is localized to the invasive tumor foci and does not indicate a preexisting underlying chronic thyroiditis (
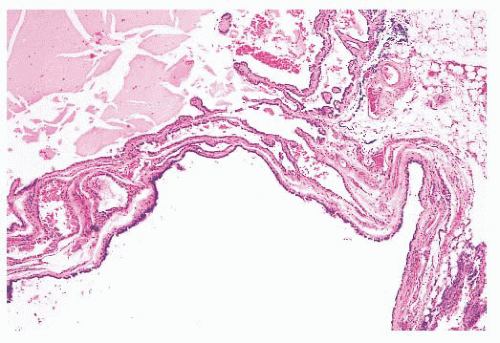
124). Cyst formation may occur and in fact may be so striking that the diagnosis of papillary carcinoma is difficult to make, particularly if the lesion has metastasized to neck lymph nodes, making the distinction (particularly on a clinical basis) from a branchial cleft cyst difficult (
135).
Papillary carcinoma invades the glandular lymphatics, which accounts for high incidence of regional node metastases (
95,
97). Papillary carcinoma can also present as multifocal tumors within the same gland. It has been shown that papillary carcinomas are clonal proliferations. The monoclonal nature of papillary carcinoma has been proven by molecular biology techniques. In view of these studies, it is believed that multifocality of papillary carcinoma must be owing to intrathyroidal lymphatic spread rather than multifocal primary tumors. Recent RET/PTC and LOH studies have shown that multifocal papillary microcarcinomas can be separate primaries instead of intraglandularly spreading from one tumor source (
114,
136). Hence, both possibilities can exist: true multiclonal lesions as well as intraglandular spread from one primary tumor.
What does this multifocality mean in regard to therapy and prognosis? Some argue that because of multifocal microscopic lesions, the entire gland should be excised; others indicate that more conservative surgery (lobectomy or lobectomy and isthmusectomy) followed by thyroid suppression is adequate because the tumors are often hormonally controllable (
137,
138). The low frequency of local recurrence in the opposite thyroid lobe and the long-term follow-up studies of conservatively treated patients showing excellent results suggest that the second viewpoint is the wiser one. Of course, treatment of extrathyroidal or massive papillary cancer requires more radical procedures (
139).
Venous invasion can be identified in up to 7% of papillary cancers (
71). It has been suggested that cases with histologic vascular invasion may be considered as a sign of an increased tendency toward hematogenic invasion and consequent increase in the relative percentage of metastases (
140).
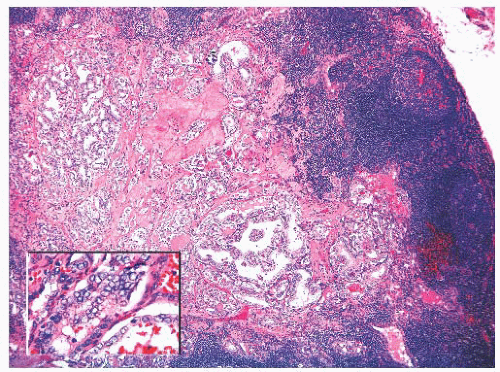
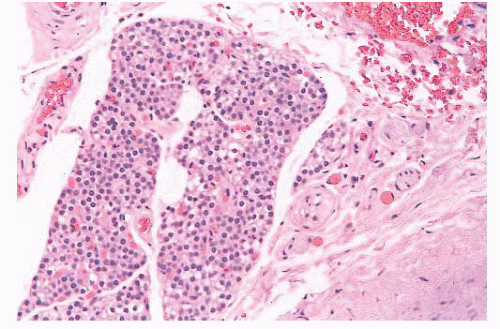
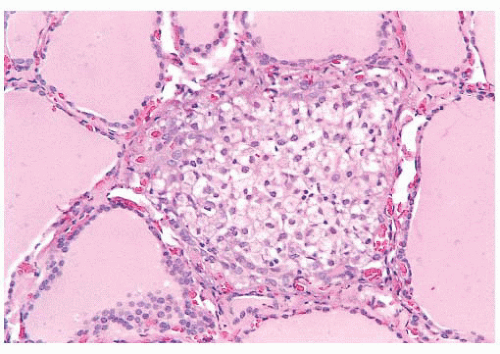
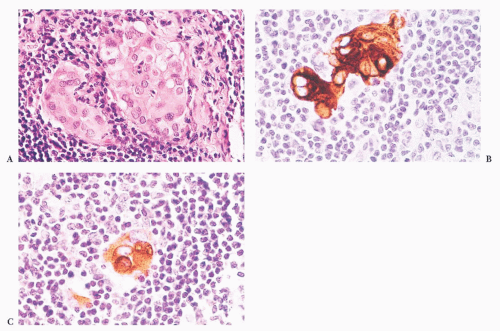
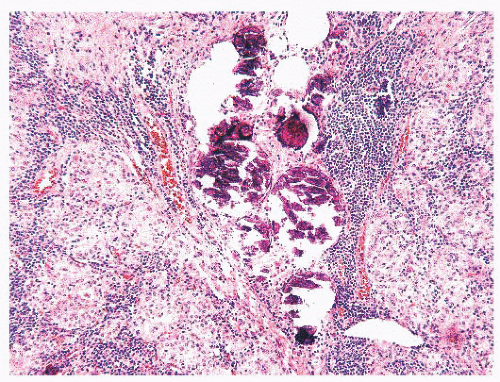
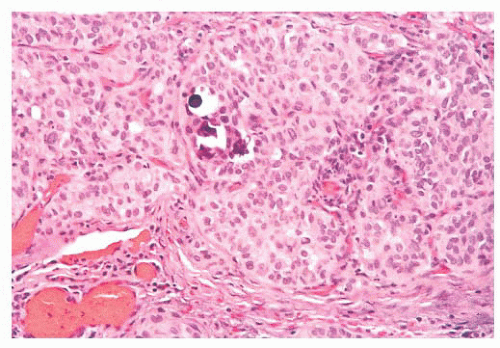
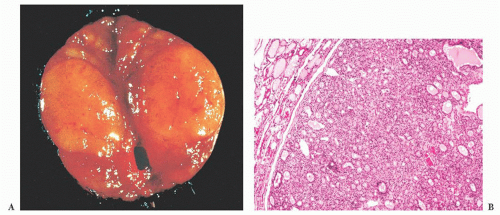
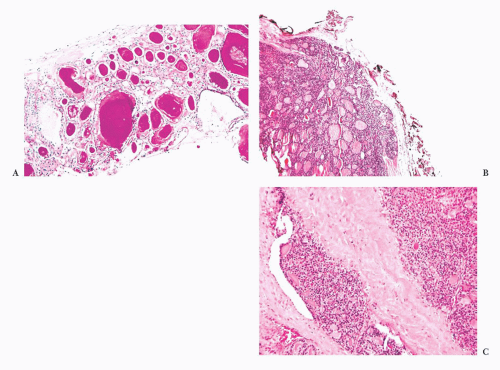
Regional lymph node metastases are extremely common (≥50%) at initial presentation of usual papillary cancer (
Fig. 13.11). This feature does not adversely affect long-term prognosis (
141,
142). Hence, attempts at staging papillary carcinoma may have minimal clinical significance. Some patients will present with cervical node enlargement and will have no obvious thyroid tumor. Frequently, the nodal metastasis will involve one node that may be cystic (
Fig. 13.12) (
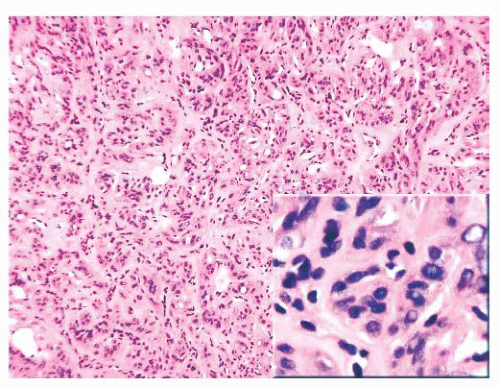
143). The histology of the nodal metastases in papillary cancer may appear papillary, mixed, or follicular (
Fig. 13.13) (
71).
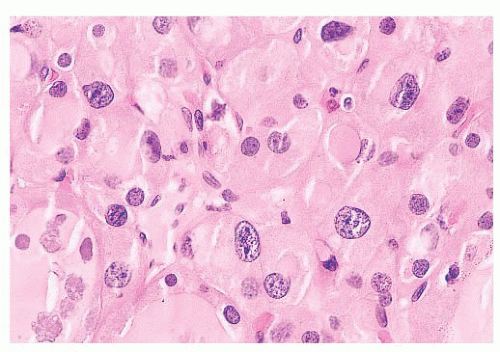
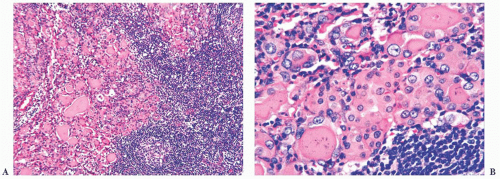
Histologic grading is of no use in papillary carcinoma because more than 95% of these lesions are grade 1. In some tumors, either in the primary site or in recurrences, areas of poorly differentiated cancer characterized by solid growth of tumor, necrosis, mitotic activity, and cytologic atypia can be found. Such lesions have a much more guarded prognosis (
125). Anaplastic change in a papillary cancer can occur, although it is uncommon (
144).
Distant metastases of papillary carcinoma to lungs and bones occur in 5% to 7% of cases (
95,
98). Despite the presence of multiple metastases, however, survival may still be prolonged, especially if the metastases can be treated with radioiodine (
145). In ordinary papillary carcinoma, death is uncommon (
95).
Molecular Pathology of Papillary Carcinoma
In the past decade, the literature on thyroid has been focused mainly on the role of various biologic events and genetic determinants in the pathogenesis of various thyroid tumors.
Rearrangements of
RET gene, known as
RET/PTC, have been identified in papillary carcinoma of thyroid (
155,
156,
157 and
158). The
RET proto-oncogene is normally expressed in cells of neural crest origin and plays a role in kidney and gastrointestinal neuronal development. It is located on chromosome 10q11.2 and cell membrane receptor tyrosine kinase. In normal thyroid, wild-type
RET is only expressed in C cells and not follicular cells.
RET/PTC seen in papillary carcinomas occurs as a result
of fusion of tyrosine kinase domain of
RET to the 5’ portion of the various genes (
155). To date, 11 novel types of rearrangements have been described in papillary carcinoma.
RET/PTC1 and
RET/PTC3 are the most common forms that occur in sporadic papillary carcinoma.
RET/PTC1 is formed by fusion of
RET to
H4, and
RET/PTC3 occurs as a result of fusion of
RET to the
ELE1 gene (
159).
RET/PTC expression in thyroid follicular cells of transgenic mice leads to development of thyroid tumors with histologic features of PTC (
160). Similarly, transfection of follicular cells in tissue culture by
RET/PTC causes the cells to demonstrate nuclear features of papillary carcinoma (
161). The prevalence of
RET/PTC in papillary carcinoma varies significantly among various geographic regions (
162,
163); in the United States it ranges from 11% to 43% (
164). In sporadic tumors,
RET/PTC1 is the most common form of rearrangement (60% to 70%), followed by
RET/PTC3 (20% to 30%) (
164). The other rare forms of
RET/PTC rearrangements have been mainly found in radiation-induced papillary carcinomas (
165). Several studies have shown a strong association between radiation-induced papillary carcinoma and expression of
RET/PTC; in papillary carcinoma, among children affected by the Chernobyl nuclear accident,
RET/PTC3 was found to be the most common form of rearrangement, followed by
RET/PTC1 (
165,
166).
Recently, it has been shown that
RET/PTC expression can also occur in some benign lesions. These include hyalinizing trabecular adenoma, Hashimoto thyroiditis, and hyperplastic nodules and follicular adenoma (
167,
168 and
169).
Several authors have suggested an association between Hashimoto thyroiditis and papillary carcinoma (
167,
168); however, others have suggested that this association is most likely incidental because both are common (
113). Recently, two independent studies have shown high prevalence of
RET/PTC in histologically benign thyroid tissue affected by Hashimoto thyroiditis; these studies concluded that thyroiditic glands harbor multiple foci of papillary carcinoma which are not identified by histologic examination only (
167,
170). However, a study by Nikiforova et al. (
113) have disputed these findings and failed to reproduce these results.
RET/PTC has been identified in benign thyroid nodules, especially the ones that are seen in patients with history of external radiation. However, the significance of this still remains controversial and needs to be further elucidated by examination of a large cohort of cases (
169).
Activation of the RAS oncogene is considered to be an important mechanism by which human cancer develops. RAS has been shown to regulate several pathways that contribute to cellular transformation including the Raf/MEK/ERK pathway. Numerous studies confirm that the Raf/MEK/ERK pathway is a significant contributor to the malignant phenotype associated with deregulated RAS signaling.
An activating mutation in
BRAF has been described in 29% to 69% of human PTCs. BRAF activating mutations in thyroid cancer are almost exclusively the
BRAF (V600E) mutation and have been found in 29% to 69% of PTCs, 13% of poorly differentiated cancers, and 10% of anaplastic cancers (
171,
172 and
173). The role of
BRAF as an oncogenic-initiating event in thyroid follicular cells has been confirmed in mice models (
174).
There is practically no concordance between papillary carcinoma with
RET/PTC, BRAF, or
RAS mutations. The lack of this overlap provides strong evidence of this signaling pathway for thyroid follicular cells to develop into papillary carcinoma (
175). Molecular analysis of fine-needle aspiration (FNA) samples for
BRAF and
RAS mutations and
RET/PTC translocations have been shown to be of value in the preoperative diagnosis of PTC in cases diagnosed as indeterminate or suspicious for malignancy (
176,
177).
Other Oncogenes.
Somatic rearrangements of
NTRK gene give rise to four chimeric genes with transforming activity; these are designated as
TRK, TRK-T1, TRK-T2, and
TRK-T3. NTRK-1 proto-oncogene has been detected in 5% to 25% of spontaneous and radiation-induced papillary carcinomas (
178,
179). The other genes implicated in the pathogenesis of PTC include
erbB-2, PTEN, p53, p16
Ink4A, and p15
Ink4B (
121,
180,
181 and
182).
Prognostic Factors
Poor prognostic factors in papillary carcinoma include older age at diagnosis, male sex, large tumor size, and extrathyroidal growth. Pathologic variables associated with a more guarded prognosis include less differentiated or solid areas, vascular invasion, and aneuploid cell population (
183,
184).
The most widely accepted system of staging solid organ malignancies is the postoperative tumor-node-metastasis (pTNM) system, endorsed by both the International Union Against Cancer (UICC) and the American Joint Commission on Cancer (AJCC). Generally, this system stages malignant lesions according to the tumor size and invasiveness, nodal spread, and distant metastases. On the basis of this AJCC staging system, all patients younger than 45 years of age with PTC or follicular thyroid cancer have stage I disease unless they have distant metastases, in which circumstance the disease is classified as stage II. Older patients (≥45 years of age) with node-negative papillary or follicular microcarcinoma (T1N0M0) have stage I disease. Intrathyroidal tumors 1.1 cm or larger are stage II, and either nodal involvement or extrathyroid invasion in older patients with papillary or follicular thyroid carcinoma leads to stage III of classification scheme (
185,
186).
Some studies have shown that
RET/PTC expression in papillary carcinoma can be associated with aggressive biologic behavior (
187), whereas others have reported just the opposite (
188): that
RET/PTC expression is more commonly seen in slow-growing and clinically indolent tumors. It is also suggested that different rearrangements of
RET/PTC are associated with different biologic behavior (
189). Nikiforov (
164) found a significant difference in local recurrence and distant metastases between tumors with
RET/PTC1 and
RET/PTC3 expression. Cetta and colleagues (
190) reported similar findings.
It has been shown that papillary carcinoma with
BRAF mutations follows an aggressive clinical course (i.e., extrathyroidal extension and more advanced clinical stage). In addition, aggressive phenotype of papillary carcinoma such as tall cell variant is associated with high prevalence of
BRAF mutations (
191).
Several other biologic markers have been suggested as prognostic predictors in papillary carcinoma; these include p53, Ki-67, cell cycle proteins, proliferating cell nuclear antigen (PCNA), bcl-2, cathepsin D, and topoisomerase II (
192,
193 and
194).