Table 18-1. Thyroid Preparations for the Treatment of Hypothyroidism
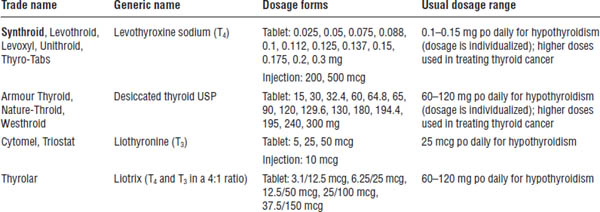
Boldface indicates one of top 100 drugs for 2012 by units sold at retail outlets, www.drugs.com/stats/top100/2012/units.
Drug interactions
■ Amiodarone may cause hypothyroidism or hyperthyroidism.
■ Antacids decrease absorption of levothyroxine. Separate administration by at least 4 hours.
■ Antidiabetic agents may be less effective with levothyroxine. An increase in the insulin or oral hypoglycemic dose may be needed.
■ Bile acid sequestrants reduce absorption of levothyroxine. Separate administration by at least 4 hours.
■ Enzyme-inducing antiepileptic agents increase hepatic degradation of levothyroxine. The thyroxine dosage may need to be increased.
■ Estrogens may decrease response to levothyroxine. The levothyroxine dosage may need to be increased.
■ Lithium commonly causes hypothyroidism.
■ Levothyroxine may enhance warfarin’s effect. The warfarin dosage may need to be decreased.
■ Levothyroxine supplementation may reduce digoxin levels.
■ Sucralfate may decrease levothyroxine absorption. Separate administration by at least 4 hours.
■ Soybean formula decreases levothyroxine absorption.
■ Sympathomimetic drugs may potentiate the effects of levothyroxine.
■ Levothyroxine may enhance theophylline clearance.
Monitoring parameters
■ Plasma TSH should be done every 6–8 weeks until normalization.
■ Signs and symptoms of hypothyroidism should improve within a few weeks.
■ Once the optimum replacement dose is attained, a physical examination should be made and TSH level should be monitored every 6–12 months.
■ Patients at risk for coronary artery disease should be monitored for angina.
Pharmacokinetics
■ The U.S. Food and Drug Administration states that all levothyroxine products should be considered therapeutically inequivalent unless equivalence (AB rating) has been established and noted in the Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations.
■ Levoxyl, Levothroid, Synthroid, Unithroid, and some generics are bioequivalent.
■ Because of the narrow therapeutic index of levothyroxine, many experts recommend rechecking TSH concentrations 6–8 weeks after any change in formulation, even when bioequivalent.
■ Oral absorption is improved by fasting but decreased by dietary fiber, drugs, and foods.
■ A half-life of 7 days allows once-daily dosing.
■ Average bioavailability of levothyroxine products ranges from 40% to 80%. When a switch is made from oral to intravenous levothyroxine, the dosage should be reduced by 25% to 50%.
Other
■ Use of natural thyroid hormones such as desiccated thyroid USP is discouraged because their potency and stability are less predictable than synthetic levothyroxine.
■ Synthetic T3 (liothyronine) has a shorter half-life than levothyroxine, has a higher incidence of cardiac side effects, and is more difficult to monitor.
Hyperthyroidism
Disease overview
Definition and epidemiology
■ Hyperthyroidism (thyrotoxicosis) is the clinical syndrome that results when tissues are exposed to high levels of thyroid hormone.
■ Thyrotoxicosis is more common in women than men, occurring in 3 per 1,000 women.
Types
■ Graves’ disease is the most common cause of hyperthyroidism.
■ Toxic multinodular goiter (MNG), toxic adenoma, and exogenous thyroid hormone ingestion may also cause hyperthyroidism.
■ Thyroid storm is a life-threatening, sudden exacerbation of all the symptoms of thyrotoxicosis, characterized by fever, tachycardia, delirium, and coma.
■ Hyperthyroidism may be caused by drugs such as amiodarone and iodine.
Clinical presentation
■ Symptoms include heat intolerance, weight loss, weakness, palpitations, and anxiety.
■ Signs include tremor; tachycardia; weakness and eyelid lag; and warm, moist skin.
■ Other manifestations include atrial fibrillation and congestive heart failure in patients with documented cardiac history.
Pathophysiology
■ Graves’ disease is an autoimmune disease in which thyroid-stimulating antibodies are produced. These antibodies mimic the action of TSH on thyroid tissue.
■ Toxic adenomas and MNGs are masses of thyroid tissue that secrete thyroid hormones independent of pituitary control.
Diagnosis
Elevated T4 or T3 in the presence of a decreased TSH confirms the diagnosis of hyperthyroidism.
Treatment principles
■ The three primary methods for controlling hyperthyroidism are surgery, radioactive iodine (RAI), and antithyroid (thioamide) drugs.
■ The goal is to minimize symptoms and eliminate excess thyroid hormone.
■ RAI is often considered the treatment of choice in Graves’ disease, toxic adenomas, and MNGs.
■ Propylthiouracil is no longer preferred in pregnancy because of the increased risk of hepatoxicity; RAI is contraindicated.
■ Thioamide drugs (propylthiouracil and methimazole) have no permanent effect on thyroid function.
■ Adjunctive treatments for hyperthyroidism include β-adrenergic receptor blockers or nondihydropyridine calcium channel blockers to control tachycardia associated with hyperthyroidism.
Drug therapy of hyperthyroidism
Antithyroid medications are summarized in Table 18-2.
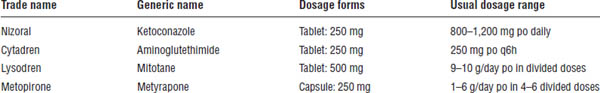
Table 18-2. Antithyroid Medications

Thioamides
Mechanism of action
■ Propylthiouracil and methimazole inhibit the synthesis of thyroid hormones by preventing the incorporation of iodine into iodotyrosines and by inhibiting the coupling of monoiodotyrosine and diiodotyrosine to form T4 and T3.
■ Propylthiouracil inhibits the peripheral conversion of T4 to T3.
Patient counseling
■ This medication prevents excessive thyroid hormone production.
■ It must be taken regularly to be effective.
■ Do not discontinue use without first consulting your physician.
■ Notify your physician if fever, sore throat, unusual bleeding, rash, abdominal pain, or yellowing of the skin occurs.
Adverse effects
■ CNS: Fever, headache, paresthesias
■ General: Rash, arthralgia, urticaria
■ GI: Jaundice, hepatitis
■ Hematologic: Agranulocytosis, leukopenia, bleeding
■ Black box warning: Propylthiouracil should be used only in patients where other treatments are not tolerated because of potentially fatal cases of severe liver injury and acute liver failure.
Drug interactions
Potentiation of warfarin’s effect may occur.
Monitoring parameters
■ Monitor for improvement in signs and symptoms of hyperthyroidism.
■ Perform thyroid function tests with periodic blood counts; watch for signs and symptoms of agranulocytosis (fever, malaise, sore throat).
Pharmacokinetics
Propylthiouracil and methimazole are typically dosed three to four times daily, but evidence exists that both drugs can be given once daily.
Iodides
Mechanism of action
■ Medication blocks hormone release and inhibits thyroid hormone synthesis.
■ Medication may be used to rapidly reduce thyroid hormone secretion when desired, such as in thyroid storm, or to decrease glandular vascularity prior to thyroidectomy.
■ Because of its harsh taste, iodine is not used for long-term thyroid suppression.
Patient instructions
■ Dilute with water or fruit juice to improve taste.
■ Notify physician if fever, skin rash, metallic taste, swelling of the throat, or burning of the mouth occurs.
Adverse effects
Adverse effects include rash, swelling of salivary glands, metallic taste, burning of the mouth, GI distress, hypersensitivity, and goiter.
Drug interactions
Lithium potentiates the antithyroid effect of iodides.
Monitoring parameters
Monitor for improvement in signs and symptoms of hyperthyroidism and for adverse effects.
18-4. Adrenals
Cushing’s Syndrome
Disease overview
Definition and epidemiology
■ Cushing’s syndrome results from chronic glucocorticoid excess.
■ The incidence rate is 2–4 persons per million population each year.
Types
■ Cushing’s syndrome is usually iatrogenic, caused by therapy with glucocorticoid drugs.
■ Endogenous Cushing’s syndrome is usually caused by overproduction of adrenocorticotropic hormone (ACTH) by pituitary gland adenomas (Cushing’s disease).
Clinical presentation
Patients may present with obesity involving the face, neck, trunk, and abdomen; hypertension; hirsutism; acne; amenorrhea; depression; thin skin; easy bruising; diabetes; and osteopenia.
Pathophysiology
The hypothalamus produces a corticotropin-releasing hormone, which stimulates the anterior pituitary gland to release ACTH (corticotropin). Circulating ACTH stimulates the adrenal cortex to produce cortisol.
Diagnosis
■ Diagnosis is usually based on signs and symptoms of hypercortisolism.
■ Dexamethasone suppression test or 24-hour urine cortisol measurement may be used.
Treatment principles
■ If the syndrome is iatrogenic, minimization of corticosteroid exposure is essential.
■ Pharmacotherapy of Cushing’s syndrome is aimed at reducing cortisol production or activity with drugs, radiation, or surgery.
Drug therapy of Cushing’s syndrome
Drugs for Cushing’s syndrome are described in Table 18-3.
Mechanism of action
■ Drugs used to treat Cushing’s disease suppress synthesis of cortisol.
■ Ketoconazole inhibits cytochrome P450 (CYP450)–dependent enzymes and cortisol synthesis.
■ Aminoglutethimide inhibits conversion of cholesterol to pregnenolone.
■ Mitotane is a cytotoxic drug that suppresses ACTH secretion and reduces synthesis of cortisol.
■ Metyrapone decreases cortisol synthesis by inhibition of 11-hydroxylase activity.
Patient counseling
Ketoconazole should be taken with food. Separate from antacids by at least 2 hours. Notify the physician if abdominal pain, yellow skin, or pale stool occurs.
Adverse effects
■ Ketoconazole causes nausea, vomiting, headache, impotence, and hepatotoxicity.
■ Aminoglutethimide causes drowsiness, rash, weakness, hypotension, nausea, loss of appetite, hypothyroidism, and blood dyscrasias.
■ Metyrapone causes nausea, vomiting, dizziness, and sedation.
■ Mitotane may cause nausea, vomiting, diarrhea, and tiredness.
Drug interactions
■ Ketoconazole is a CYP450 3A4 enzyme inhibitor and may increase serum concentrations of cyclosporine, warfarin, cisapride, and triazolam. Drugs that lower gastric acidity will decrease ketoconazole absorption. Rifampin decreases ketoconazole levels.
■ Aminoglutethimide may induce metabolism of warfarin.
Monitoring parameters
Cortisol monitoring is required with mitotane.
Adrenal Insufficiency
Disease overview
Definition and epidemiology
■ Primary adrenocortical deficiency (Addison’s disease) is caused by autoimmune-mediated destruction of the adrenal cortex and results in glucocorticoid and mineralocorticoid deficiency.
■ Addison’s disease occurs in 5–6 persons per million population per year.
Types
■ Primary adrenal insufficiency (Addison’s disease) involves autoimmune destruction of the adrenal cortex.
Table 18-3. Drugs for Cushing’s Syndrome
■ Secondary adrenal insufficiency occurs after cessation of chronic exogenous corticosteroid use.
■ Acute adrenal insufficiency, or Addisonian crisis, is an endocrine emergency precipitated by severe stress.
Clinical presentation
■ Glucocorticoid deficiency (weight loss, malaise, abdominal pain, depression)
■ Mineralocorticoid deficiency (dehydration, hypotension, hyperkalemia, salt craving)
Pathophysiology
■ Cortisol is synthesized in the adrenal cortex when cholesterol is converted to pregnenolone by ACTH.
■ The adrenal cortex secretes aldosterone, cortisol, and androgenic hormones.
■ Mineralocorticoids (e.g., aldosterone) enhance reabsorption of sodium and water from the distal tubule of the kidney and increase urinary potassium excretion.
■ Glucocorticoids affect glucose, carbohydrate, and fat metabolism; produce anti-inflammatory and immunosuppressive effects; and affect other physiologic processes.
■ Chronic administration of corticosteroids produces inhibition of pituitary ACTH secretion and reduced cortisol production (hypothalamic-pituitary-adrenocortical [HPA] axis suppression).
■ Abrupt cessation of steroids may precipitate adrenal insufficiency.
Diagnosis
■ A cosyntropin (ACTH) stimulation test may be used to assess hypocortisolism.
Treatment principles
■ Addison’s disease requires lifelong glucocorticoid and mineralocorticoid replacement.
■ Hydrocortisone 100 mg intravenous (IV) q8h is the drug of choice for acute adrenal crisis.
■ “Stress doses” of corticosteroids are given for minor illness, injury, or surgery. If stress is severe, hydrocortisone 100 mg IV q8h is used.
■ Gradual tapering of corticosteroids reduces the risk of adrenal insufficiency in patients with HPA axis suppression.
■ Nonadrenal uses for corticosteroids are numerous, including allergic reactions; inflammatory conditions; hematologic disorders; rheumatic disorders; neurologic diseases; cancer; immunosuppression; pulmonary, renal, skin, and thyroid diseases; and hypercalcemia.
■ Fludrocortisone has minimal anti-inflammatory activity and is used only when mineralocorticoid activity is needed, such as when increased blood pressure is desired.
Drug therapy of adrenal insufficiency
Information about corticosteroids is provided in Table 18-4.
Table 18-4. Corticosteroids and Dose Equivalents
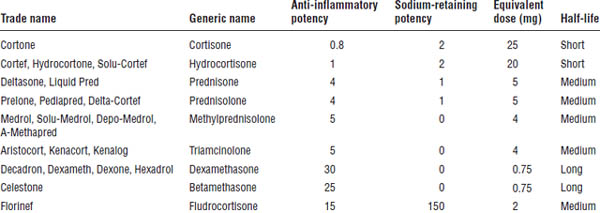
Mechanism of action
■ Glucocorticoids increase blood glucose by stimulating gluconeogenesis and glycogenolysis; fat deposition is increased.
■ Catabolic effects occur in lymphoid, connective tissue, bone, muscle, fat, and skin.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


