(1)
Department of Pathology, Medical College of Wisconsin, Milwaukee, Wisconsin, USA
Keywords
ThymomaThymic epithelial neoplasmsAtypical thymomaThymic carcinomaThymic epithelial neoplasms are the most common primary tumors of the anterior mediastinum. The terminology and classification of these tumors have been a topic of debate for many years and have continued to evolve as we have gained a better understanding of their pathogenesis and biologic behavior. Thymic epithelial neoplasms comprise a spectrum of lesions that can range from very low-grade, well-differentiated tumors to high-grade, poorly differentiated neoplasms, with a wide range of morphologies in-between. The most widely used classification is that proposed by the World Health Organization (WHO), which consists of several categories and utilizes mostly a combination of numbers and letters to designate the various types; however, a more simplified classification scheme has been proposed by Suster and Moran that stratifies these tumors into three categories based on their degrees of differentiation and clinical behavior (Table 2.1). A confusing situation also exists with the persistent use of the terms “thymoma” and “thymic carcinoma” to refer to the ends of the morphologic spectrum in these tumors, implying that one is malignant and the other benign, when in reality all primary thymic epithelial neoplasms have the potential for aggressive or malignant behavior irrespective of their histologic appearance. For the time being, we will utilize the term “thymic carcinoma” in this chapter for tumors that are cytologically obviously malignant, following the standard and traditional nomenclature utilized by the majority of investigators and reserve the term “thymoma” for those tumors that fall mainly in the low-grade and intermediate range of the morphologic spectrum.
Well- and moderately differentiated (low- and intermediate-grade) tumors are traditionally designated as thymomas. These tumors represent slow-growing, low- to intermediate-grade neoplasms that retain at least some of the organotypical features of the normal thymus (e.g., lobulation, biphasic cell population composed of thymic epithelial cells admixed with lymphocytes, and dilated perivascular spaces). The tumors are often bulky and usually surrounded by a thick fibrous capsule that can contain calcifications. They usually manifest in the anterosuperior mediastinum, although rare cases can occur in ectopic locations, including the lung and perihilar, pleural, and posterior mediastinal locations. Thymomas are associated with paraneoplastic syndromes in up to 50 % of cases, particularly in myasthenia gravis but also in aplastic anemia, essential thrombocytosis, hemolytic anemia, hypogammaglobulinemia, mixed collagen-vascular diseases, myopathies, pure red-cell aplasia, systemic lupus erythematosus, and others. Thymomas can also undergo progression to malignancy and give rise to full-blown thymic carcinoma arising from a preexisting, more benign-appearing thymoma.
Poorly differentiated (high-grade) primary thymic epithelial neoplasms are designated by the conventional name of thymic carcinoma and are seldom associated with myasthenia gravis or other paraneoplastic syndromes. These tumors usually manifest a much more aggressive behavior and have a generally poor prognosis despite aggressive therapy. Thymic carcinomas histologically resemble their counterparts in other organs (i.e., squamous cell, adeno-, clear cell, spindle-cell, and anaplastic carcinoma). A large number of histologic variants have been described, some of which have shown a tendency to be associated with secondary cystic changes such as basaloid and mucoepidermoid carcinoma. The diagnosis of thymic carcinoma is, in general, a diagnosis of exclusion, since there is very little that is distinctive or pathognomonic about these tumors other than their location in the mediastinum. Morphologically, it can be very difficult to separate them from similar cancers occurring at other organs.
Table 2.1
Current histologic classifications of thymic epithelial neoplasms
Suster and Moran (1999) | WHO (2015) |
|---|---|
Well-differentiated thymic epithelial neoplasms (thymoma) | Thymoma type A Thymoma type AB |
Moderately differentiated thymic epithelial neoplasms (atypical thymoma) | Thymoma type B1 Thymoma type B2 Thymoma type B3 |
Poorly differentiated thymic epithelial neoplasms (thymic carcinoma) | Special types of thymoma (various) |
Thymic carcinoma |
2.1 Thymomas
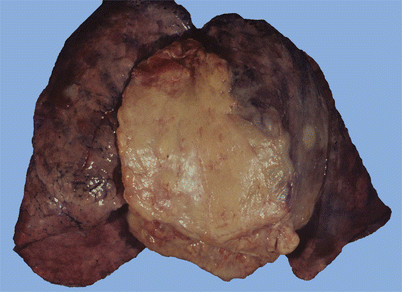
Fig. 2.1
Thymoma at autopsy; incidental finding in a 54-year-old man who died of complications of hypertension and pulmonary embolism shows a large, fleshy, anterior mediastinal mass that is focally infiltrating adjacent pleura and lung parenchyma
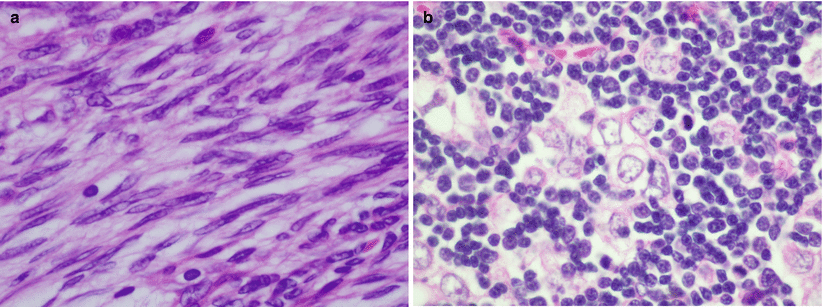
Fig. 2.2
Cytologic appearance of the two basic types of thymoma in the WHO classification: (a) WHO type A is composed of elongated cells with spindle or oval nuclei, dispersed nuclear chromatin, and an inconspicuous rim of cytoplasm. The cells are devoid of atypia. (b) Thymomas WHO type B are composed of cells with round vesicular nuclei with prominent, single eosinophilic nucleoli surrounded by an ample rim of lightly eosinophilic or amphophilic cytoplasm. Notice the abundance of small lymphocytes surrounding the larger epithelioid cells
2.1.1 Spindle-Cell Thymoma (WHO Type A)
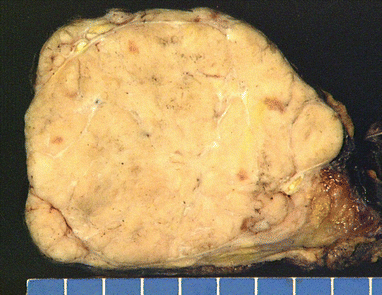
Fig. 2.3
Gross appearance of spindle-cell thymoma (WHO type A) shows a nodular fleshy appearance with homogeneous rubbery tissue. Notice that the tumor is well circumscribed and surrounded by a thin fibrous capsule
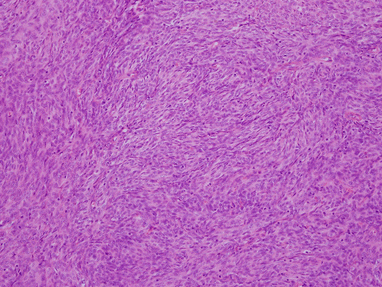
Fig. 2.4
Typical histologic appearance of spindle-cell thymoma (WHO type A) shows a fascicular proliferation of spindle cells arranged in short fascicles with no discernible atypia. A few scant small lymphocytes are seen scattered among the spindle cells
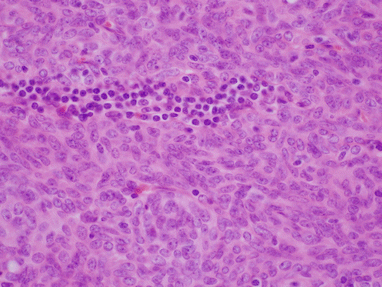
Fig. 2.5
Higher magnification of spindle-cell thymoma shows sheets and fascicles of small, bland-appearing spindle cells containing scattered small lymphocytes
Table 2.2
Histologic variants of spindle-cell (WHO type A) thymoma
Fascicular |
Storiform |
Whorling (meningioma-like) |
Rosette forming |
Glandular |
Papillary |
Sclerosing |
Adenoid |
Micro- and macrocystic |
Hemangiopericytic |
Thymoma with trabecular, ribbon-like, and neuroendocrine-like growth patterns |
Thymoma with pseudosarcomatous stroma (metaplastic thymoma) |
Micronodular thymoma with B-cell hyperplasia |
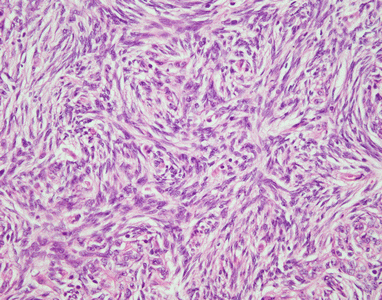
Fig. 2.6
Spindle-cell thymoma with storiform pattern. The tumor is characterized by short storiform structures resembling the pattern of growth of cutaneous dermatofibrosarcoma protuberans. Notice, however, the bland appearance of the spindle cells with a sprinkling of small lymphocytes. Tumors with these features can be mistaken for sarcomas of fibrohistiocytic type or solitary fibrous tumors
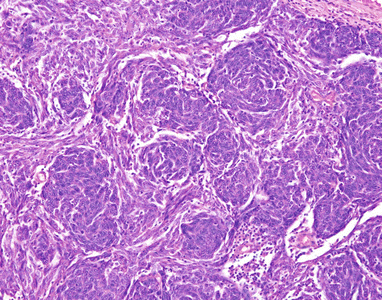
Fig. 2.7
A spindle-cell thymoma with a whorling pattern shows discrete rounded areas composed of concentrically aligned spindle cells superficially resembling meningothelial whorls
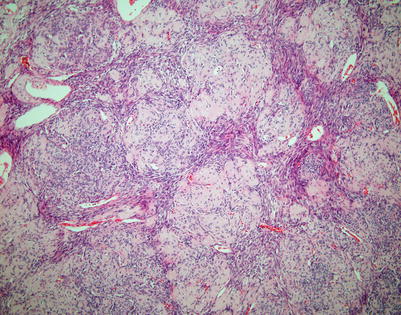
Fig. 2.8
Variation in the theme of spindle-cell thymoma with whorling pattern showing gradual sclerosis and hyalinization of the cellular whorls, resulting in a giant rosette-like structure
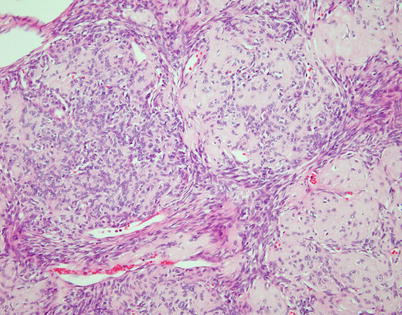
Fig. 2.9
Higher magnification of the preceding image shows discrete, rounded areas of stromal sclerosis containing a sparse population of small round to oval cells surrounded by fascicles of spindle cells
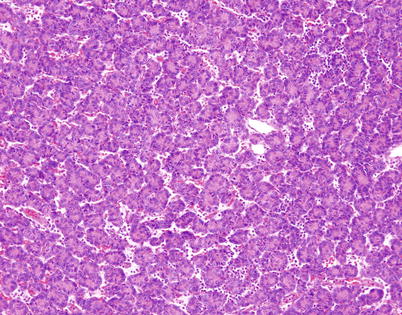
Fig. 2.10
Spindle-cell thymoma with prominent rosette-like structures. The tumor shows numerous small, round structures containing spindle to oval nuclei showing a palisaded arrangement toward the periphery of the rosette-like structures that is reminiscent of neuroblastic and primitive neuroectodermal neoplasms
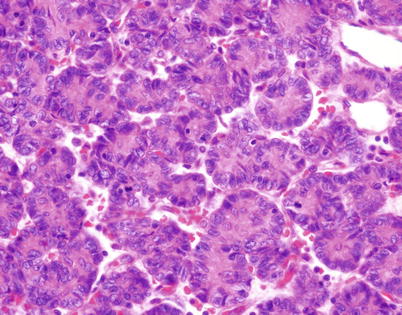
Fig. 2.11
Higher magnification of rosette-like structures in spindle-cell thymoma. The central portions of the structures contain eosinophilic material that resembles the fibrillary material in Homer-Wright rosettes. Thymomas with these features can be confused with metastases from neuroblastoma and other neuroectodermal neoplasms or with neuroendocrine neoplasms (carcinoid tumors and large cell neuroendocrine carcinoma). The cells in these structures stain positive for cytokeratin and p63 but are unreactive to neuroendocrine and neural markers
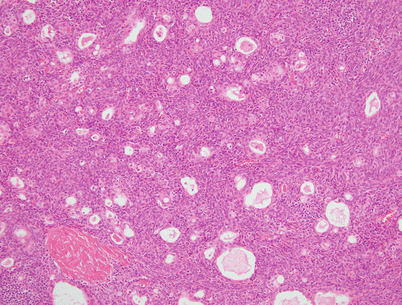
Fig. 2.12
Spindle-cell thymoma with numerous small gland-like spaces, some of which are filled with eosinophilic proteinaceous material. The gland-like spaces can impart to the lesion a biphasic appearance that is reminiscent of biphasic synovial sarcoma
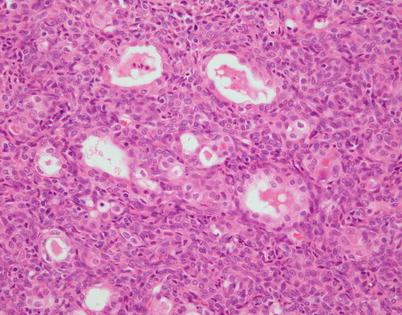
Fig. 2.13
Higher magnification of spindle-cell thymoma with gland-like structures. The gland-like structures are lined by a layer of large, epithelioid cells resembling a glandular lining. Notice the monotonous spindle cells surrounding the gland-like structures; both stained positive with cytokeratin and p63
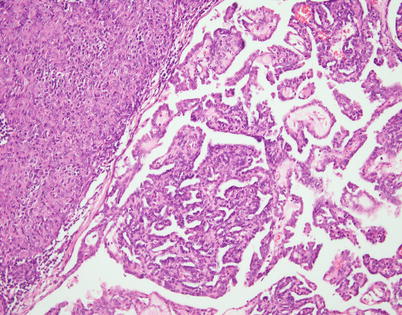
Fig. 2.14
Spindle-cell thymoma with papillary architecture. Notice the solid spindle-cell thymoma component (top left) that transitions abruptly into areas with well-developed papillary tufts protruding into empty spaces
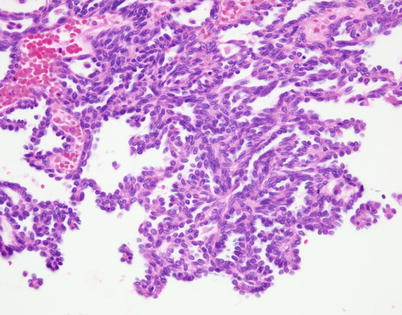
Fig. 2.15
Higher magnification of spindle-cell thymoma with papillary architecture. Notice that the cells populating the stroma of the papillae are oval- to spindle-appearing and look bland. Tumors with similar features but with invasive properties have been described as “papillary thymic carcinoma”
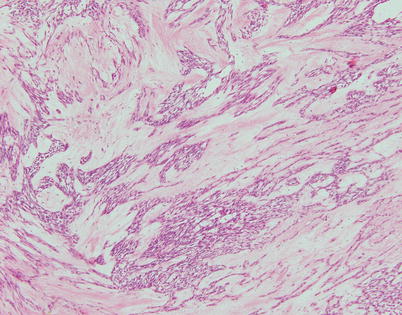
Fig. 2.16
Spindle-cell thymoma with stromal sclerosis. The tumor shows strands of spindle thymic epithelial cells embedded in an abundant fibrocollagenous matrix. Tumors with these features can be mistaken for schwannian neoplasms with prominent stromal degenerative changes
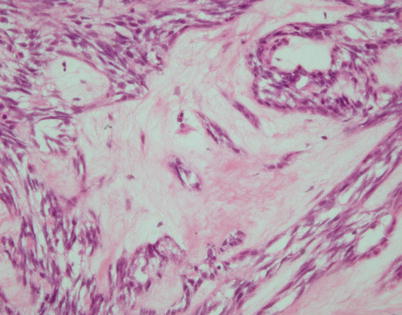
Fig. 2.17
Higher magnification of spindle-cell thymoma with stromal hyalinization shows that the areas of stromal sclerosis correspond to hyalinized dilated perivascular spaces
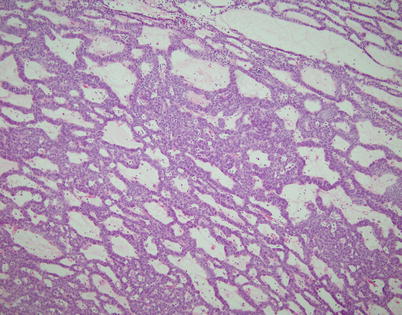
Fig. 2.18
Spindle-cell thymoma with “adenoid” growth pattern. The tumor resembles a skin adnexal tumor and is composed of thin trabeculae of round to oval tumor cells growing with a sieve-like architecture with intervening loose myxoid stroma
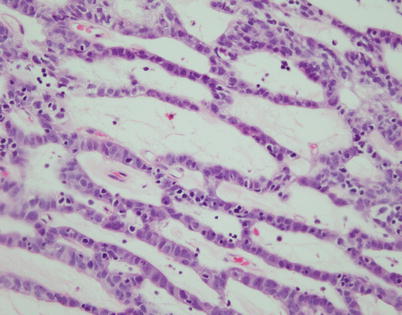
Fig. 2.19
Higher magnification from spindle-cell thymoma with adenoid growth pattern shows large, round to oval tumor cells with abundant cytoplasm admixed with a sprinkling of small lymphocytes. Notice that the intervening gland-like spaces correspond to dilated perivascular spaces with stromal hyalinization
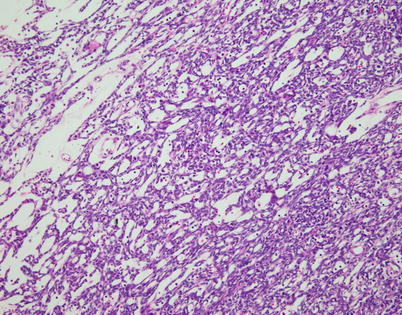
Fig. 2.20
Spindle-cell thymoma can often undergo cystic degenerative changes. In this particular example, the tumor is characterized by multiple small cystic spaces, resulting in a reticular pattern of growth
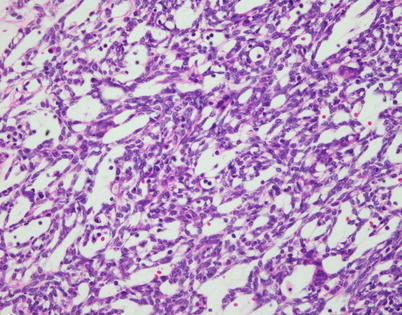
Fig. 2.21
Spindle-cell thymoma with reticular and microcystic growth patterns. Notice that the cells in the walls of the cysts are small and spindled and there are scattered small lymphocytes admixed with the cells and in the cystic lumina
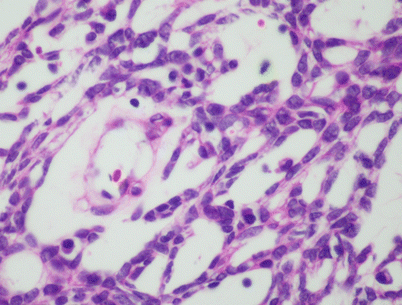
Fig. 2.22
Spindle-cell thymoma with microcystic growth pattern. Higher magnification shows that the microcystic spaces correspond to abortive perivascular spaces. Notice the central vessel in the larger cyst on the left
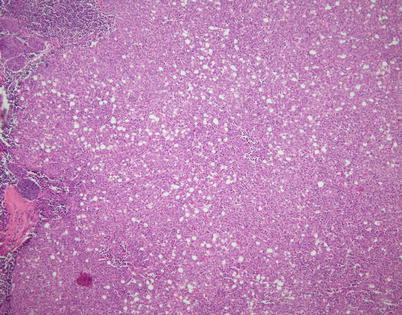
Fig. 2.23
Another variation on the topic of microcystic thymoma shows a dense spindle-cell proliferation dotted by numerous small empty spaces suggestive of adipocytes on scanning magnification. Notice the scattering of small lymphocytes admixed with the spindle cells in the background
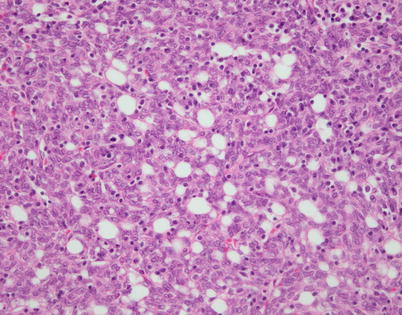
Fig. 2.24
Higher magnification from spindle-cell thymoma with microcysts shows small empty lumina scattered among the spindle cells that contain a rare small lymphocyte. The cells lack the features of adipocytes but may also not be recognized as perivascular spaces because of the absence of a centrally located vessel
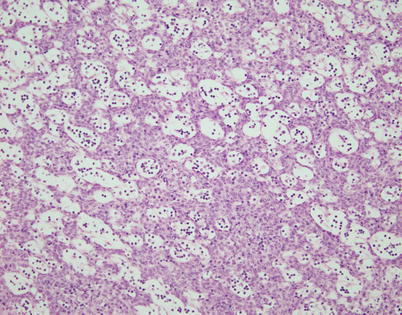
Fig. 2.25
Another aspect of spindle-cell thymoma with a microcystic growth pattern shows evenly spaced small cystic cavities surrounded by bland-appearing monotonous spindle-cell proliferation
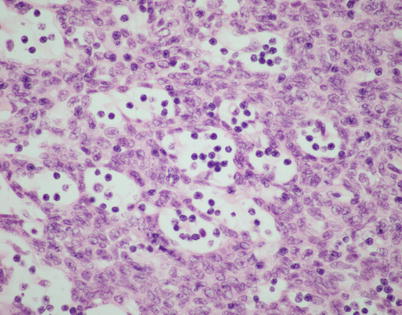
Fig. 2.26
Higher magnification from microcystic spindle-cell thymoma shows that the small cystic spaces contain small lymphocytes
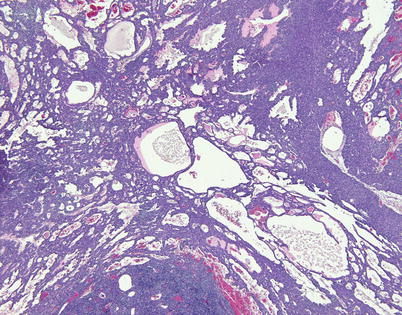
Fig. 2.27
A spindle-cell thymoma with a macrocystic growth pattern shows numerous small dilated perivascular spaces that have focally coalesced to form larger, macrocystic spaces. The process can advance to form a large multicystic mass that can be grossly confused for a multilocular thymic cyst
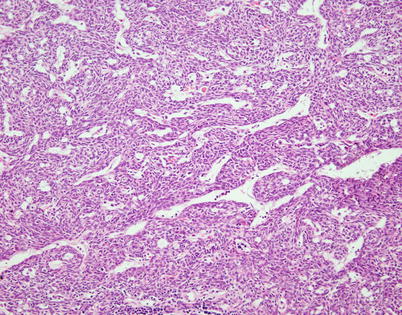
Fig. 2.28
A spindle-cell thymoma with a hemangiopericytoma-like growth pattern is characterized by multiple branching vascular spaces with open lumina surrounded by a dense spindle-cell population. On cursory examination, tumors with these features can be mistaken for solitary fibrous tumors or monophasic synovial sarcomas
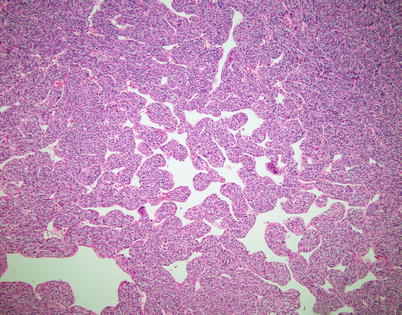
Fig. 2.29
A different appearance of spindle-cell thymoma with a hemangiopericytoma-like growth pattern shows dilated, anastomosing, and branching vascular spaces lined by round to oval cells simulating hemangiopericytoma
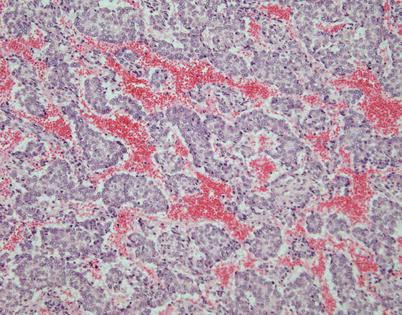
Fig. 2.30
Trabecular growth pattern in spindle-cell thymoma shows elongated strands of cells that are several layers thick separated by vascular stroma simulating a neuroendocrine neoplasm. The tumor cells in this case were p63 positive and negative for neuroendocrine markers
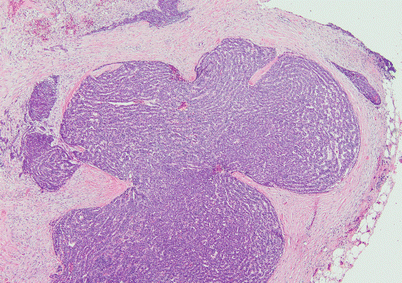
Fig. 2.31
Ribbon-like growth pattern in spindle-cell thymoma shows a lobulated growth surrounded by connective tissue that contains serpiginous cords of tumor cells; the pattern can be highly reminiscent of a neuroendocrine neoplasm such as thymic carcinoid
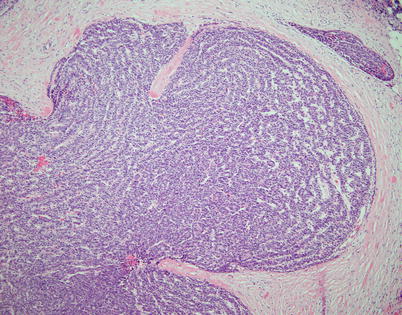
Fig. 2.32
Higher magnification from ribbon-like growth pattern in spindle-cell thymoma shows spindle to oval cells with hyperchromatic nuclei aligned in parallel arrays forming serpiginous cords of cells
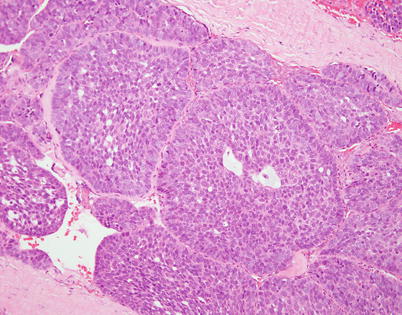
Fig. 2.33
Spindle-cell thymoma showing prominent basaloid peripheral palisading of nuclei similar to that observed in large cell neuroendocrine carcinomas of the lung. The nuclear morphology, however, is bland and does not show the stippling of chromatin or prominent nucleoli of large cell neuroendocrine carcinoma
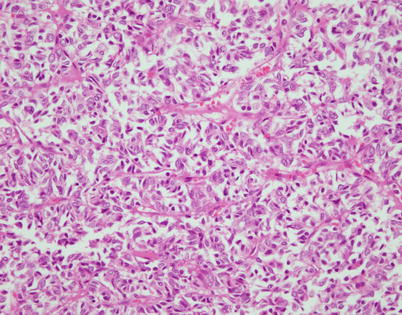
Fig. 2.34
Spindle-cell thymoma showing a subtle “tzellballen” pattern composed of small nests of cells separated by fibrovascular stroma. Such cases need to be distinguished from mediastinal paragangliomas, well-differentiated neuroendocrine carcinoma, and metastatic melanoma
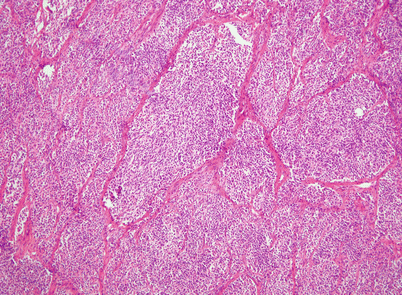
Fig. 2.35
Another histologic appearance in spindle-cell thymoma that can be confused with metastases of melanoma or primary neuroendocrine carcinoma is characterized by large clusters of spindle cells displaying a prominent nesting pattern
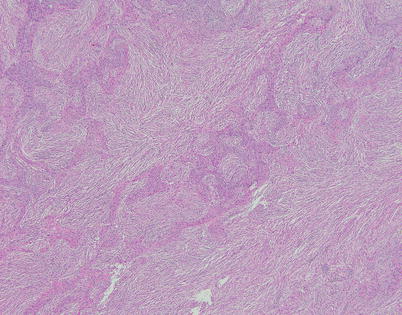
Fig. 2.36
Spindle-cell thymoma with pseudosarcomatous stroma (the so-called “metaplastic” thymoma) is an unusual variant characterized by anastomosing strands and islands of cohesive epithelioid cells separated by a highly cellular spindle-cell stroma simulating a biphasic malignant neoplasm (i.e., carcinosarcoma)
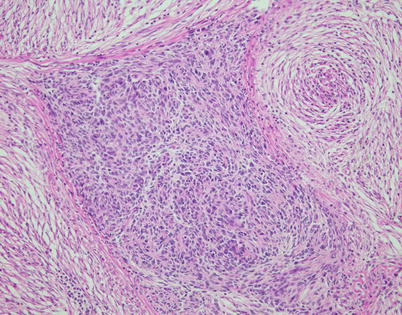
Fig. 2.37
Spindle-cell thymoma with pseudosarcomatous stroma shows a discrete island composed of oval to polygonal epithelioid cells with minimal cytologic atypia surrounded by a cellular spindle-cell stroma. The epithelial cells test positive for cytokeratins, p63, and PAX8; the stromal spindle cells are positive for vimentin and may also display focal weak actin and EMA positivity
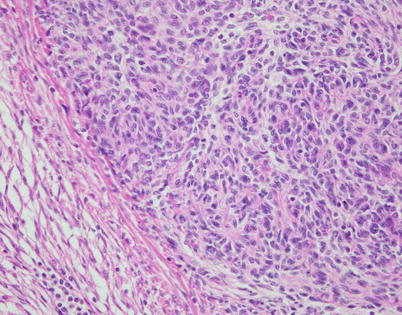
Fig. 2.38
Higher magnification of epithelial cell island in spindle-cell thymoma with pseudosarcomatous stroma shows cells with oval nuclei and abundant eosinophilic cytoplasm; note that the cells are devoid of mitotic activity
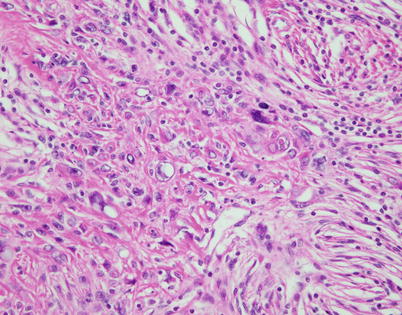
Fig. 2.39
Focal areas in spindle-cell thymoma with pseudosarcomatous stroma may display squamoid features and degenerative atypia; these features should not be confused with evidence of malignancy or the development of thymic carcinoma
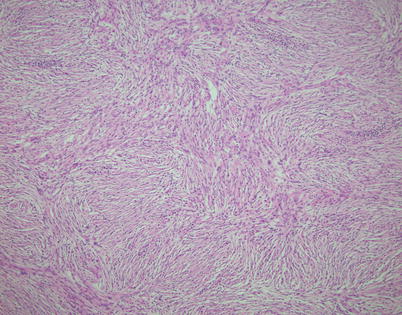
Fig. 2.40
The stromal component in spindle-cell thymoma with pseudosarcomatous stroma is characterized by a bland population of spindle cells devoid of any significant cytologic atypia, nuclear pleomorphism, or mitotic activity that often adopts a fascicular or storiform pattern
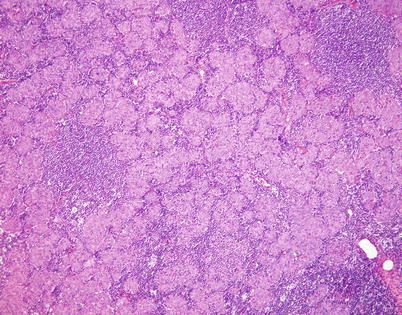
Fig. 2.41
Micronodular thymoma corresponds to an unusual variant of spindle-cell thymoma characterized by a distinct nodular growth pattern with dense lymphoid stroma. Unlike the lymphoid stroma in conventional thymomas, the small lymphocytes in these tumors are composed of reactive, polyclonal B cells rather than immature T cells
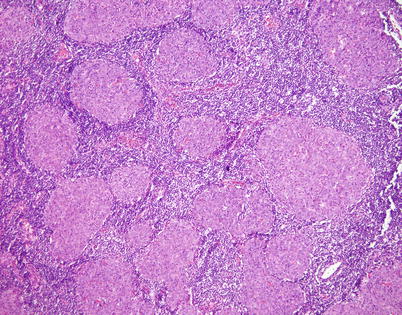
Fig. 2.42
Higher magnification of micronodular spindle-cell thymoma with B-cell hyperplasia shows nodules of varying sizes composed of oval to spindle cells with abundant eosinophilic cytoplasm. The stroma surrounding the nodules is densely packed with a polyclonal population of B lymphocytes admixed with plasma cells
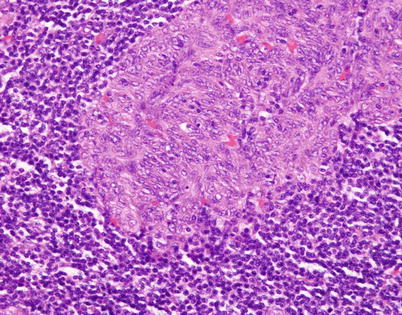
Fig. 2.43
Higher magnification in micronodular spindle-cell thymoma shows an island of epithelial cells characterized by oval to spindle nuclei without significant cytologic atypia and devoid of mitotic activity. The cells are indistinguishable from those of conventional spindle-cell thymoma and are positive for cytokeratin, p63, and PAX8
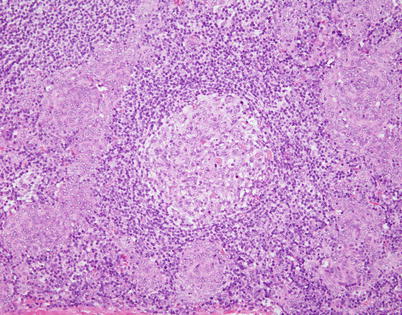
Fig. 2.44
Micronodular spindle-cell thymoma can also contain scattered hyperplastic lymphoid follicles with reactive germinal centers (center). Rare cases of lymphoma arising from micronodular thymoma have been described
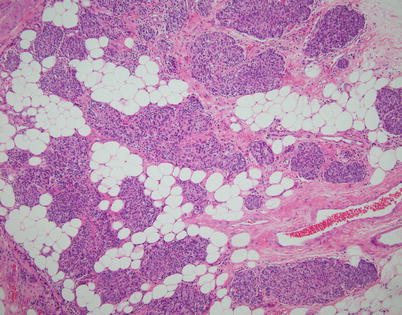
Fig. 2.45
Invasive spindle-cell thymoma shows infiltration of perithymic fat by tumor with through-and-through penetration of the capsule. Spindle-cell thymomas are also capable of invasion of surrounding structures and metastases and can be associated with aggressive behavior in a small percentage of cases (~10 %). In such cases, prognosis is dictated by the staging of the tumor independent of the histology
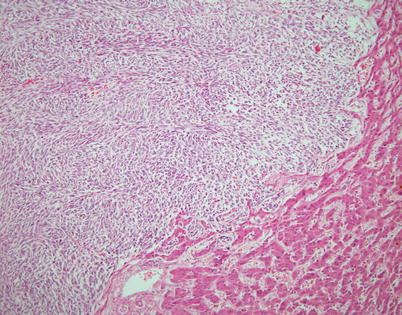
Fig. 2.46
Liver metastasis of spindle-cell thymoma discovered at autopsy in a 54-year-old woman who died as a result of widespread metastases from an invasive spindle-cell thymoma
2.2 Lymphocyte-Rich Spindle-Cell Thymoma (WHO Type AB)
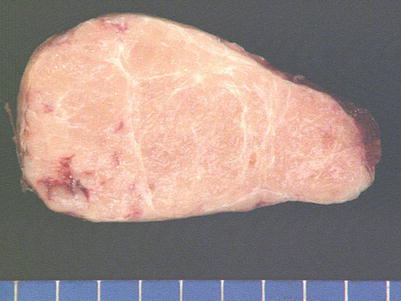
Fig. 2.47
Gross appearance of lymphocyte-rich spindle-cell thymoma (WHO type AB) shows a well-circumscribed and encapsulated mass with a tan-white lobulated cut surface
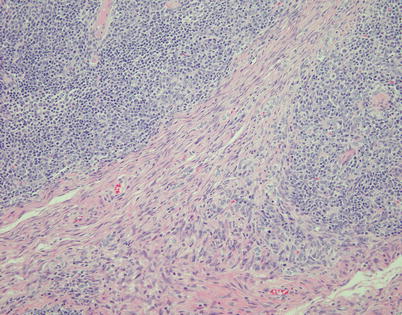
Fig. 2.48
Lymphocyte-rich spindle-cell thymoma (WHO type AB) is characterized by areas displaying conventional spindle-cell thymoma (WHO type A, lower half) that is poor in lymphocytes admixed with areas that contain abundant lymphocytes (type B-like areas, top right and left). Although in the original definition of the WHO this tumor was supposed to contain an admixture of type A and type B cells (i.e., spindle/oval cells and round/epithelioid cells), in reality this is a misconception because the shape of the cells is the same in both the lymphocyte-poor and the lymphocyte-rich areas
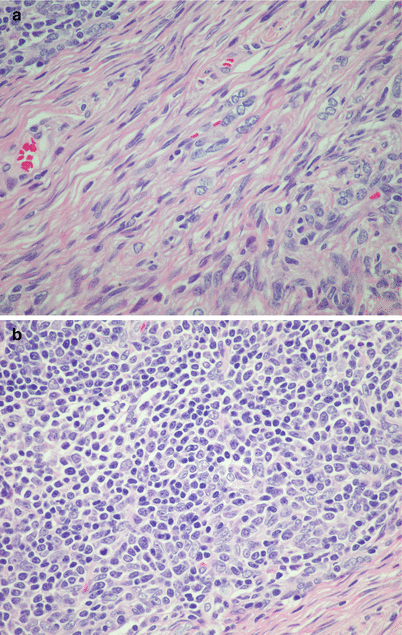
Fig. 2.49




Higher magnification from preceding field (type AB thymoma) showing (a) lymphocyte-poor spindle-cell thymoma component (WHO type A) with oval to spindle nuclei without any discernible cytoplasm and dispersed chromatin with inconspicuous nucleoli and (b) lymphocyte-rich component (B-like component) showing an abundance of scattered small lymphocytes admixed with oval to spindle nuclei without any discernible cytoplasm and dispersed chromatin with inconspicuous nucleoli (i.e., the thymic epithelial cells are the same in both components, and there are no type B cells)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


