Table 41-1. Recommendations for VTE Prophylaxis
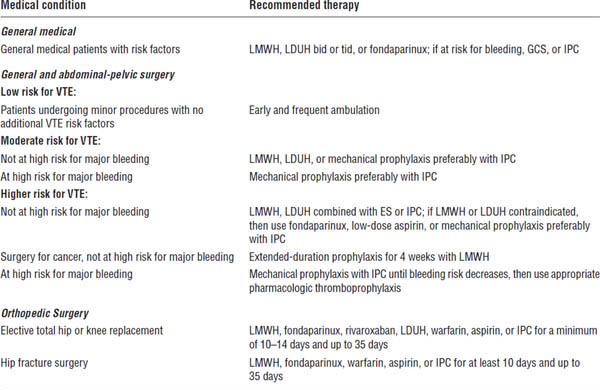
Ageno, Gallus, Wittkowsky, et al., 2012.
LMWH, low molecular weight heparin: enoxaparin 30 mg subcutaneous q12h, enoxaparin 40 mg subcutaneous q24h, dalteparin 5,000 units subcutaneous q24h; LDUH, low-dose unfractionated heparin, 5,000 units subcutaneous q8–12h; fondaparinux 2.5 mg subcutaneous q24h; GCS, graduated compression stocking; IPC, intermittent pneumatic compression device; ES, elastic stocking; warfarin, target INR (international normalized ratio) 2.5, range 2.0–3.0.
Table 41-2. Guidelines for Anticoagulation: IV Unfractionated Heparin
Indication | Guidelines |
VTE suspected | Obtain baseline aPTT, PT, and CBC count. |
| Check for contraindications to heparin therapy. |
| Order imaging study; administer IV bolus of heparin 80 IU/kg or 5,000 IU. |
VTE confirmed | Rebolus with IV heparin 80 IU/kg, and start maintenance infusion at 18 IU/kg/h. |
| Check aPTT at 6 hours to keep aPTT in a range that corresponds to a therapeutic blood heparin level. |
| Check platelet count between days 1 and 3. |
| Start warfarin therapy on day 1 at 5 mg, and adjust subsequent daily dose according to INR. |
| Stop heparin after at least 4–5 days of combined therapy when INR is ≥ 2.0 for 24 hours. |
| Anticoagulate with warfarin for at least 3 months at an INR of 2.5; range of 2.0–3.0. |
Adapted with permission from Kearon, Kahn, Agnelli, et al., 2008.
CBC, complete blood count; IV, intravenous; INR, international normalized ratio; PT, prothrombin time.
For treatment with subcutaneous UFH, give 250 IU/kg subcutaneous q12h to obtain a therapeutic aPTT in 6 hours.
Table 41-3. Guidelines for Anticoagulation: LMWH or Fondaparinux
Indication | Guidelines |
VTE suspected | Obtain baseline aPTT, PT, and CBC. |
| Check for contraindications to LMWH or fondaparinux therapy. |
| Order imaging study; administer LMWH or fondaparinux. |
VTE confirmed | Continue LMWH or fondaparinux. |
| Check platelet count between days 3 and 5. |
| Start warfarin therapy on day 1 at 5 mg, and adjust subsequent daily dose according to INR. |
| Stop LMWH or fondaparinux after at least 4–5 days of combined therapy when INR is ≥ 2.0 for 24 hours. |
| Continue warfarin for at least 3 months at an INR of 2.5; range of 2.0–3.0. |
Adapted with permission from Kearon, Kahn, Agnelli, et al., 2008.
CBC, complete blood count; INR, international normalized ratio; PT, prothrombin time.
Dalteparin sodium 200 anti Xa IU/kg per day subcutaneous; single dose should not exceed 18,000 IU. Enoxaparin 1 mg/kg subcutaneous q12h or 1.5 mg/kg subcutaneous q24h; single dose should not exceed 180 mg. Fondaparinux 5 mg for < 50 kg; 7.5 mg for 50–100 kg; 10 mg for > 100 kg subcutaneous q24h.
With outpatient DVT treatment, the diagnosis may take place in either a physician’s office or the emergency department. After a Doppler ultrasound confirms the diagnosis of a DVT, the patient is educated on administration of LMWH and receives the first dose of LMWH at that time. LMWH and warfarin are then administered on an outpatient basis. Warfarin is monitored with an international normalized ratio (INR) at 1- or 3-day intervals. After two therapeutic INRs, the LMWH may be discontinued; warfarin is continued for at least 3 months, and the patient is evaluated for long-term anticoagulation or as indicated (Table 41-4). Another therapy is rivaroxaban, an oral factor Xa inhibitor. Rivaroxaban is effective within 2–4 hours, so no other therapies such as LMWH or heparin are necessary. For acute DVT or PE, rivaroxaban is administered 15 mg bid with food for 21 days followed by 20 mg daily with food.
Drug Therapy
Unfractionated heparin
Mechanism of action
UFH binds to antithrombin (AT) and converts it from a slow progressive thrombin inhibitor to a rapid thrombin inhibitor. This, in turn, catalyzes inactivation of factors XIIa, XIa, IXa, Xa, and IIa (thrombin).
Therapeutic use
■ Prevention and treatment of VTE
■ Prevention of VTE in patients with a previous VTE or a known hypercoagulability
■ Prophylaxis for VTE in high-risk populations
■ Prevention of a mural thrombosis after myocardial infarction (MI)
Table 41-4. Duration of Anticoagulation Therapy
Indication | Duration of anticoagulation |
VTE secondary to transient risk factor | Warfarin therapy for 3 months |
First unprovoked VTE | Warfarin therapy for at least 3 months, but consider long-term therapy based on risk–benefit ratio |
Second unprovoked VTE | Warfarin therapy long term |
VTE and cancer | LMWH for the first 3–6 months of long-term anticoagulation therapy, followed by anticoagulation with LMWH or warfarin therapy long term or until cancer resolves |
Adapted with permission from Kearon, Kahn, Agnelli, et al., 2008.
■ Treatment of patients with unstable angina and MI
■ Prevention of acute thrombosis after coronary thrombolysis
Patient counseling
Patients need to monitor for signs and symptoms of bleeding or bruising, especially at surgical sites.
Parameters to monitor
Heparin is monitored by an aPTT, which is sensitive to the inhibitory effects of heparin on factors IIa (thrombin), IXa, and Xa.
The College of American Pathologists and the American College of Chest Physicians (ACCP) recommend against the use of a fixed aPTT therapeutic range of 1.5–2.5 times a control aPTT. They do recommend that a therapeutic aPTT range be established on the basis of an antifactor Xa concentration of 0.3–0.7 units/mL.
An aPTT should be measured 6 hours after a bolus dose of heparin or after any dosage change and then every 6 hours until a therapeutic aPTT is reached. Once a therapeutic aPTT is achieved, an aPTT may be evaluated every 24 hours.
Platelet count and hematocrit should be evaluated at baseline and every 1–3 days.
Pharmacokinetics
The pharmacokinetics of heparin differ depending on whether an intravenous (IV) or subcutaneous route of administration is used.
Heparin is cleared from the body by a rapid saturable mechanism that occurs at therapeutic doses. A second, slower unsaturable first-order clearance that is largely by renal means occurs at high doses.
The half-life of heparin varies from approximately 30 minutes after an IV bolus of 25 IU/kg to 60 minutes after an IV bolus at 100 IU/kg.
Dosing
Heparin should be dosed using a weight-based nomogram. A therapeutic range for heparin is determined by an antifactor Xa chromogenic assay of 0.3–0.7 IU/mL. Weight-based dosing nomograms are effective in achieving a therapeutic aPTT, although they are not universally transferable to every hospital. Published nomograms are specific only for the reagent and instrument used to validate that nomogram.
Determining a therapeutic range by using the calculation of 1.5–2.5 times the mean control aPTT may be erroneous. Previous weight-based nomograms, which used a therapeutic range based on the calculation of 1.5–2.5 times the control aPTT, have been recognized to be accurate only for that aPTT reagent used. Table 41-5 is an example of a weight-based dosing nomogram. Each hospital should develop its own nomogram based on its therapeutic range.
Another approach to heparin therapy is to administer an IV bolus of 5,000 IU followed by a continuous infusion of at least 30,000–35,000 IU over 24 hours. The infusion rate is adjusted to maintain a therapeutic aPTT.
UFH may be administered subcutaneously every 12 hours. The patient should receive an initial dose of 17,500 IU or 250 IU/kg subcutaneous q12h. The dose should be adjusted to an aPTT that corresponds to a plasma heparin level of antifactor Xa chromogenic assay of 0.3–0.7 IU/mL. This level should be measured 6 hours after the injection.
Alternatively, UFH may be administered as a fixed dose that is unmonitored. An initial dose of 333 IU/kg is administered, followed by 250 IU/kg subcutaneous every 12 hours.
Adverse effects
The most common adverse effects are minor bleeding in the form of gingival bleeding, epistaxis, and ecchymosis. The most common serious adverse effects of heparin are gastrointestinal or urogenital bleeding.
Fatal or life-threatening adverse effects often result from intracranial or retroperitoneal bleeding.
Transient thrombocytopenia may occur within the first 2–4 days of therapy, which will resolve with continued therapy. Heparin-induced thrombocytopenia may also occur, which requires discontinuation of the heparin.
Osteoporosis is a risk with chronic use.
Table 41-5. Body Weight–Based Dosing of IV Heparin
aPTT | Dose |
Initial dose | 80 IU/kg bolus, then 18 IU/kg/h |
< 35 | 80 IU/kg bolus, then 4 IU/kg/h |
35–45 | 80 IU/kg bolus, then 2 IU/kg/h |
46–70a | No change |
71–90 | Decrease infusion rate by 2 IU/kg/h |
> 90 | Hold infusion 1 hour, then decrease infusion rate by 3 IU/kg/h |
Adapted from Raschke, Gollihare, Peirce, 1996.
a. The therapeutic aPTT range of 46–70 seconds corresponded to antifactor Xa activity of 0.3–0.7 IU/mL at the time this study was performed. The therapeutic range at any institution should be established by correlation with antifactor Xa levels in this range.
Table 41-6. LMWH and Pentasaccharide Dosage Forms
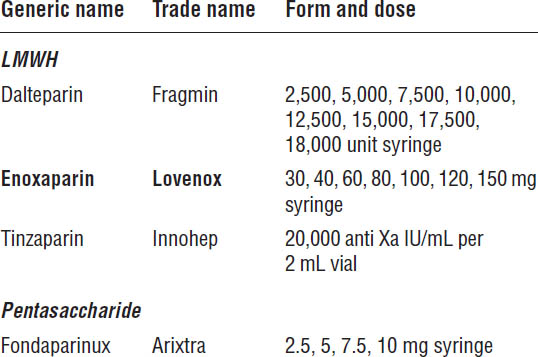
Boldface indicates one of top 100 drugs for 2012 by units sold at retail outlets, www.drugs.com/stats/top100/2012/units.
Contraindications
Contraindications include the following:
■ Active bleeding
■ Severely uncontrolled hypertension
■ History of heparin-induced thrombocytopenia
Use epidural or spinal anesthesia with caution because patients are at risk of developing an epidural or spinal hematoma, which can result in long-term or permanent paralysis.
LMWHs and pentasaccharide
Table 41-6 describes LMWH and pentasaccharide dosage forms. The pharmacokinetics of LMWHs and pentasaccharides are described in Table 41-7.
Mechanism of action
LMWHs inhibit factor Xa and, to a much lesser extent, factor IIa.
Table 41-7. Pharmacokinetics of LMWHs and Pentasaccharides
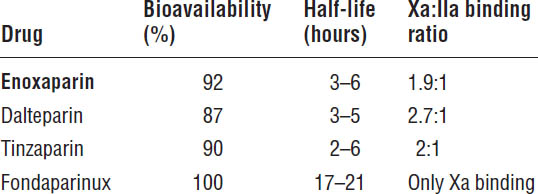
Boldface indicates one of top 100 drugs for 2012 by units sold at retail outlets, www.drugs.com/stats/top100/2012/units.
Fondaparinux is a pentasaccharide. It binds selectively to AT, which potentiates the inactivation of factor Xa in the coagulation cascades, thus inhibiting the formation of thrombin.
Advantages of LMWH and fondaparinux over UFH
■ LMWHs and fondaparinux have fewer interactions with plasma proteins; thus, they have a more predictable response at lower doses.
■ LMWHs have a much longer half-life, which allows them to be administered subcutaneously every 12–24 hours.
■ Fondaparinux has a half-life of 17–21 hours, which allows dosing every 24 hours.
■ LMWHs have a lower incidence of osteoporosis and heparin-induced thrombocytopenia. Fondaparinux does not cause heparin-induced thrombocytopenia and can be used as an anticoagulant to treat heparin-induced thrombocytopenia.
Therapeutic use
■ Prevention and treatment of VTE
■ Prevention of VTE in patients with a previous VTE or a known hypercoagulability
■ Prophylaxis for VTE in high-risk populations
■ Arterial embolism prevention in patients with mechanical or tissue prosthetic heart valve replacement
■ Arterial embolism prevention in patients with atrial fibrillation or atrial flutter
■ Arterial embolism prevention in patients with an acute cardioembolic stroke
Patient counseling
■ Strict compliance is necessary to ensure a consistent level of anticoagulation.
■ Notify a health care provider if bruising, hematuria, melena, hemoptysis, epistaxis, gingival bleeding, or any other abnormal bleeding occurs.
■ Consult a health care provider or pharmacist before taking any over-the-counter medications.
■ Avoid aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs).
■ The air bubble in the LMWH or fondaparinux syringe should be near the plunger before injection. This method ensures that all of the drug is expelled from the syringe and helps minimize the amount of bleeding, bruising, and hematoma formation from the injection site.
Parameters to monitor
Monitor platelet counts, hematocrit and hemoglobin, and signs and symptoms of bleeding. Bone mineral density should be monitored with long-term use.
Anti Xa heparin levels can be monitored in obese patients or in patients receiving LMWH who have significant renal impairment. Anti Xa levels should be drawn 4 hours after a dose. Therapeutic levels are 0.6–1 IU/mL for twice-daily dosing and 1–2 IU/mL for once-daily dosing. The therapeutic range for dalteparin is 0.5–1.5 IU/mL 4–6 hours after receiving 3–4 doses.
Prothrombin time (PT)/INR and aPTT are not useful in monitoring LMWH.
Currently, fondaparinux has no direct monitoring parameters.
Dosing LMWH and fondaparinux
See Tables 41-8 and 41-9 for dosage requirements.
Adverse effects
The most common adverse effects are minor bleeding in the form of gingival bleeding, epistaxis, and ecchymosis. The most common serious adverse effects of heparin are gastrointestinal or urogenital bleeding.
Fatal or life-threatening adverse effects often result from intracranial or retroperitoneal bleeding.
Heparin-induced thrombocytopenia can occur with LMWH, but its incidence is greater with UFH.
Osteoporosis can occur with chronic use.
Direct thrombin inhibitors
Argatroban
Mechanism of action
Argatroban is a synthetic molecule that reversibly binds to thrombin.
Therapeutic use
Argatroban is used for prophylaxis and treatment of thrombosis associated with heparin-induced thrombocytopenia and percutaneous coronary intervention.
Table 41-8. Indications and Recommended Doses of LMWH and Fondaparinux
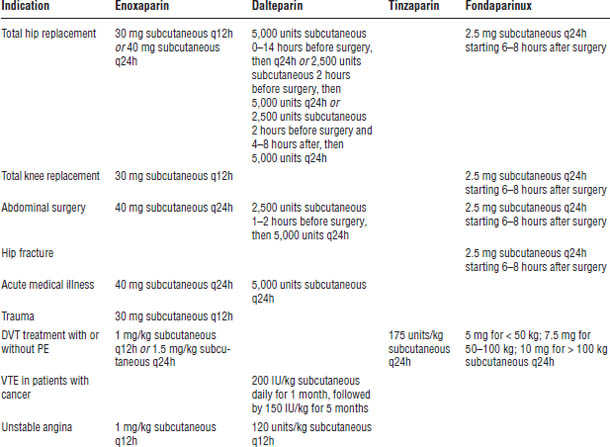
Enoxaparin is one of top 100 drugs for 2012 by units sold at retail outlets, www.drugs.com/stats/top100/2012/units.
Table 41-9. Enoxaparin Dosage Regimens for Patients with Severe Renal Impairment (Creatinine Clearance < 30 mL/min)

Enoxaparin is one of top 100 drugs for 2012 by units sold at retail outlets, www.drugs.com/stats/top100/2012/units.
Patient counseling
Monitor symptoms of bruising and bleeding, and report them to a health care provider immediately.
Parameters to monitor
Monitor complete blood count (CBC) and signs and symptoms of bleeding. The aPTT is used to monitor and to adjust argatroban therapy. The aPTT should be drawn 2 hours after an infusion is started and after each dosage change.
Argatroban will also elevate a PT/INR. For patients on concomitant warfarin therapy, this effect may impede monitoring and make proper assessment of the INR difficult. For combination therapy, argatroban may be discontinued after an INR is > 4. An INR should be drawn after 4–6 hours. If the INR is in the therapeutic range, continue with warfarin only. If the INR is below the therapeutic range, restart argatroban and increase the dose of warfarin. Repeat this procedure until the INR is within the therapeutic range.
Pharmacokinetics
Argatroban is metabolized in the liver to inactive metabolites. The half-life is 0.5–1 hour.
Dosing
Administer a continuous IV infusion at the rate of 2 mcg/kg/min. Adjust infusion rate to maintain an aPTT ratio of 1.5–2.5. The usual dose is 2–10 mcg/kg/min.
The dose should be reduced in moderate hepatic insufficiency. A continuous IV infusion should begin at a rate of 0.5 mcg/kg/min. Because of the prolonged elimination half-life, measure the aPTT 4 hours after initiation or a dosage change.
Adverse effects
The most common adverse effects are minor bleeding in the form of gingival bleeding, epistaxis, and ecchymosis. The most common serious adverse effects are gastrointestinal or urogenital bleeding.
Fatal or life-threatening adverse effects often result from intracranial or retroperitoneal bleeding.
There is no known antidote for argatroban. The anticoagulant effect declines rapidly after discontinuation of the drug.
Nonhemorrhagic effects such as fever, nausea, vomiting, and allergic reactions rarely occur.
Warfarin (Coumadin)
Dosage forms
■ Tablets: 1 mg (pink), 2 mg (lavender), 2.5 mg (green), 3 mg (tan), 4 mg (blue), 5 mg (peach), 6 mg (teal), 7.5 mg (yellow), 10 mg (white)
■ Injections (IV): 5 mg powder for reconstitution (2 mg/mL)
Mechanism of action
Warfarin is a vitamin K antagonist that produces its pharmacologic effect by interfering with the interconversion of vitamin K and its 2,3-epoxide (vitamin K epoxide). Warfarin leads to the depletion or reduction in activity of vitamin K–dependent coagulation proteins (factors II, VII, IX, and X) produced in the liver. The level and activity of the vitamin K–dependent clotting factors decline over 6–96 hours. At least 4–5 days of warfarin therapy are necessary before a patient is completely anticoagulated.
Therapeutic use
■ Prevention and treatment of VTE
■ Prevention of VTE in patients with a previous VTE or a known hypercoagulability
■ Prophylaxis for VTE in high-risk populations
■ Prevention of arterial embolism in patients with mechanical or tissue prosthetic heart valve replacement
■ Prevention of arterial embolism in patients with atrial fibrillation or atrial flutter
■ Prevention of arterial embolism in patients with a previous cardioembolic stroke
■ Prevention of acute MI in patients with peripheral arterial disease
Patient counseling
■ Warfarin should be taken at the same time every day.
■ Strict compliance is necessary to ensure a consistent level of anticoagulation.
■ Strict compliance with a consistent vitamin K diet is necessary to ensure a consistent level of anticoagulation.
■ Notify the health care provider in the event of hematuria, melena, epistaxis, hemoptysis, increased bruising, or any abnormal bleeding.
■ Notify all health care providers, including dentists, of warfarin therapy.
■ Blood monitoring to determine an adequate level of anticoagulation and compliance is necessary at regular intervals.
■ Consult a health care provider or pharmacist before taking any new prescription or over-the-counter medications.
■ Avoid aspirin or NSAIDs unless instructed otherwise by a health care provider.
■ Women of childbearing age should use an effective form of birth control because warfarin has teratogenic effects.
Parameters to monitor
Warfarin therapy is monitored by a PT. The PT responds to a reduction in factors II, VII, and X. The INR is used to standardize the responsiveness of thromboplastin to the anticoagulant effects of warfarin. The INR is calculated by the following equation:
INR = (observed PT/mean normal PT)ISI
where ISI = International Sensitivity Index, which is a measure of thromboplastin sensitivity. The lower the ISI, the more responsive the thromboplastin is to the anticoagulant effects of warfarin.
The ACCP Evidence-Based Clinical Practice Guidelines (9th edition) recommends two intensities of anticoagulation: a less intense level with a target INR of 2.5 and a range of 2.0–3.0 and a high-intensity level of anticoagulation with a target INR of 3.0 and a range of 2.5–3.5 (Table 41-10).
Upon initiation of warfarin therapy, the INR should be evaluated daily if the patient is in the hospital and every 2–3 days if the patient is not hospitalized.
Table 41-10. Recommended Therapeutic Ranges for Warfarin
Indication | Target INR (INR range) |
Prophylaxis of VTE | 2.5 (2.0–3.0) |
Treatment of VTE | 2.5 (2.0–3.0) |
Prevention of thromboembolic | 2.5 (2.0–3.0) |
Prevention of arterial embolism | 2.5 (2.0–3.0) |
Prevention of arterial embolism | 2.5 (2.0–3.0) |
Prevention of arterial embolism | 3.0 (2.5–3.5) |
In patients with a mechanical |
|
Ageno, Gallus, Wittkowsky, et al., 2012. VKA, vitamin K antagonist.
Pharmacokinetics
Warfarin is a racemic mixture of two optically active isomers, warfarin S and warfarin R, in roughly equal amounts. The S isomer is three to four times more potent than the R isomer.
Warfarin is rapidly and completely absorbed from the gastrointestinal tract with peak concentration in approximately 90 minutes. Warfarin is 99% protein bound with a half-life of 36–42 hours.
The onset of anticoagulation occurs after 4–5 days of therapy and is caused by the depletion of the clotting factors rather than steady-state concentrations of warfarin. Thus, the onset of action is based on the half-life of the clotting factors II, VII, IX, and X. The S isomer of warfarin is metabolized by cytochrome (CYP) 2C9 and, to a lesser extent, by CYP3A4. The R isomer is metabolized by CYP1A2 and CYP3A4 and, to a lesser extent, by CYP2C19.
Dosing
Time in the therapeutic range and intensity of anticoagulation are critical for optimizing the therapeutic efficacy of warfarin and minimizing the risk of hemorrhage.
Warfarin initiation does not require loading. Loading doses can result in an inappropriate increase in the INR, which is not reflective of an anticoagulant effect.
Initiating warfarin at 5 mg daily should result in an INR around 2.0 in 4–5 days for most patients. An alternative method is to administer between 5 and 10 mg for the first 1–2 days and then adjust the dose depending on the INR response.
Initiating warfarin at a dose of ≤ 5 mg daily may be appropriate in the elderly; in patients with liver disease, heart failure, or malnutrition; in patients taking drugs known to increase the responsiveness to warfarin; or in patients with a high risk of bleeding.
Initiating warfarin at a dose of 7.5–10 mg daily may be appropriate for young, healthy, or obese patients.
If a rapid anticoagulant effect is indicated, IV heparin, LMWH, or fondaparinux should be administered along with warfarin for at least 4–5 days until a therapeutic INR is reached. Heparin, LMWH, or fondaparinux may be discontinued when the INR is within the therapeutic range on two consecutive occasions.
Disease state interaction
Disease states that can increase the response to warfarin are hyperthyroidism, congestive heart failure, liver disease, fever, and genetic increased warfarin sensitivity.
Disease states that can decrease the response to warfarin are hypothyroidism and genetic warfarin resistance. Patient nonadherence can also result in a reduced warfarin response.
Drug–drug interactions
Warfarin is a drug with a narrow therapeutic index. Numerous drugs interact with warfarin. Drugs that either inhibit or induce CYP2C9 or 3A4 and, to a lesser extent, CYP1A2 or 2C19, potentiate or reduce the anticoagulant effect. The S isomer is more active than the R isomer; thus, drugs that inhibit or induce the S isomer will have a more significant effect on warfarin than drugs that inhibit or induce the R isomer (Table 41-11).
Drug–food interactions
Foods that contain high amounts of vitamin K can reduce the anticoagulant effect of warfarin (Table 41-11). It is important that patients be consistent in their consumption of these foods and that they evenly space their consumption over a 7-day period. If a patient suddenly stops eating these foods, the INR may dramatically increase.
Adverse effects
The most common adverse effect is minor bleeding in the form of gingival bleeding, epistaxis, and ecchymosis. The most common serious adverse effects of warfarin are either gastrointestinal or urogenital bleeding.
Table 41-11. Drugs and Foods That Can Interact with Warfarin
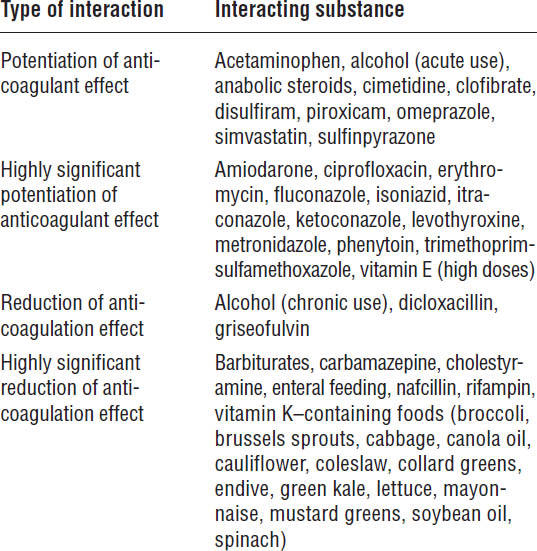
Warfarin-induced skin necrosis is a rare, but serious adverse effect. Skin necrosis begins within 10 days of warfarin initiation. It is characterized by painful, erythematous lesions on breast, thighs, and buttocks, which may progress to hemorrhagic lesions. It may be associated with protein C deficiency and, to a lesser effect, protein S deficiency. The concomitant use of UFH, LMWH, or fondaparinux with initiation of warfarin can prevent its occurrence.
Purple toe syndrome is a dark blue-tinged discoloration of the feet that occurs rarely 3–8 weeks after warfarin initiation.
Fatal or life-threatening adverse effects are related to intracranial or retroperitoneal bleeding.
Several treatment options are available for the reversal of anticoagulation. Treatment may be necessary for a supratherapeutic INR or before an invasive procedure. Simply withholding warfarin will reduce the level of anticoagulation. For an INR of 6 to 10, an estimated 2.5 days may be needed for an INR to be reduced below 4. Phytonadione, which is also referred to as vitamin K, will reverse the effects of warfarin. Phytonadione may be administered orally or IV. If phytonadione is administered IV, it should be diluted in at least 50 mL of IV fluid and administered over 20 minutes to minimize the risk of anaphylactic reactions. It should not be administered intramuscularly because of hematoma formation or subcutaneously because of erratic absorption. Administration of oral phytonadione together with withholding of warfarin therapy may result in a reduction in an INR of 6–10 to an INR of < 4 in approximately 1.4 days. IV phytonadione begins reversing an INR within 2 hours. Other treatment options for reversing an INR include fresh frozen plasma, nonactivated prothrombin complex concentrate (PCC), or recombinant factor VII. PCC is available as a three factor or four factor concentrate. Three factor PCC contains factors II, IX, and X, whereas four factor concentrate contains factors II, VII, IX, and X.
Special precautions must be taken for patients undergoing invasive procedures. Table 41-12 outlines the risk stratifications for perioperative arterial or venous thromboembolism. Box 41-1 outlines the recommendations for managing anticoagulation in these patients.
Rivaroxaban (Xarelto)
Dosage forms
■ Tablets: 10, 15, 20 mg
Mechanism of action
Rivaroxaban is a factor Xa inhibitor and does not require cofactors such as antithrombin for activity. Factor Xa is involved in both the intrinsic and the extrinsic coagulation pathways.
Therapeutic use
■ DVT/PE treatment
■ Reduction in the risk of recurrence of DVT/PE
■ Reduction of the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation
■ DVT prophylaxis in patients undergoing hip or knee replacement surgery
Patient counseling
■ Rivaroxaban is used for the treatment of a DVT or PE or to reduce the risk of the recurrence of a DVT or PE.
■ Rivaroxaban lowers the risk of developing a blood clot after hip or knee replacement surgery.
■ Rivaroxaban should be taken daily with food in patients with a DVT or PE or once daily at the same time of the day with or without food for prevention of a blood clot after hip or knee replacement surgery.
■ Failure to take rivaroxaban on a consistent basis greatly increases the chance of developing a blood clot.
■ Strict compliance is necessary to ensure a consistent level of anticoagulation.
■ If you forget to take the medication, take it as soon as you remember the same day. Do not take two doses to make up for the missed dose.
Table 41-12. Suggested Patient Risk Stratification for Perioperative Arterial or Venous Thromboembolism
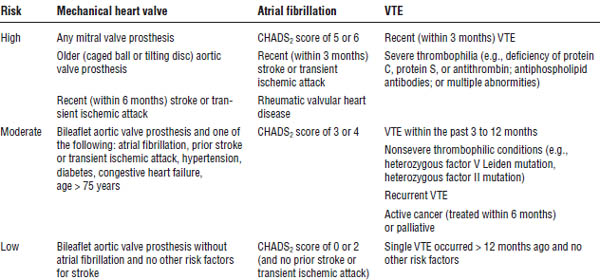
Gage, Waterman, Shannon, et al. 2001.
CHADS2, congestive heart failure (1 point), hypertension (1 point), age (1 point), diabetes (1 point), stroke (2 points).
Box 41-1. Recommendations for Managing Anticoagulation Therapy in Patients Requiring Invasive Procedures
Patients with Low Risk of Thromboembolism
■ Discontinue warfarin 5 days before procedure.
■ Consider bridging with low-dose subcutaneous LMWH.
■ Suggest therapeutic-dose subcutaneous LMWH over other management options.
Patients with Moderate Risk of Thromboembolism
■ Discontinue warfarin 5 days before procedure.
■ Consider bridging with therapeutic-dose subcutaneous LMWH or IV UFH or with low-dose subcutaneous LMWH over not bridging during interruption of warfarin therapy.
■ Suggest therapeutic-dose subcutaneous LMWH over other management options.
Patients with High Risk of Thromboembolism
■ Discontinue warfarin 5 days before procedure.
■ Bridge with therapeutic-dose subcutaneous LMWH or IV UFH during interruption of warfarin therapy.
■ In patients whose INR is still elevated (> 1.5) 1 to 2 days before surgery, administer 1 to 2 mg oral vitamin K to normalize INR.
■ Discontinue therapeutic-dose LMWH 24 hours before procedure, and administer half the total daily dose as the last preoperative dose.
■ Discontinue UFH approximately 4–6 hours before a procedure.
■ Suggest LMWH over IV UFH.
■ Restart warfarin 12–24 hours after procedure or when hemostasis is adequate.
■ Resume therapeutic-dose LMWH approximately 24 hours after the procedure or when hemostasis is adequate.
■ In patients at high bleeding risk, delay therapeutic-dose LMWH or UFH for 48–72 hours or administer low-dose LMWH or UFH when hemostasis is secured, or completely avoid LMWH or UFH.
■ Individualize treatment plans based on postoperative hemostasis and bleeding risk.
Patients Undergoing Minor Dental or Dermatologic Procedure or Cataract Removal
■ Continue warfarin therapy around the time of the procedure.
■ Co-administer an oral prohemostatic agent such as tranexamic acid or epsilon amino caproic acid mouthwash for dental procedures.
Adapted with permission from Douketis, Berger, Dunn, et al., 2008.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


