Thoracoabdominal Aortic Aneurysm Repair
Richard P. Cambria
Robert S. Crawford
Introduction
Aneurysms that simultaneously involve the thoracic and abdominal aorta and/or those aneurysms including the visceral aortic segment are referred to as thoracoabdominal aortic aneurysms (TAAAs). Such aneurysms are uncommon, comprising no more than 2% to 5% of the total spectrum of degenerative aortic aneurysm. Studies examining natural history data for thoracic aortic aneurysms indicate that between 20% and 30% of patients will also be found to have aneurysms of the abdominal aorta. Successful management of abdominal aneurysm involving the visceral aortic segment was first performed by Etheredge in 1954. The modern era in TAAA management began with the pioneering work of E. Stanley Crawford, who described a simplified operative approach, the “clamp and sew” method, which also consisted of the inclusion technique wherein visceral and intercostal vessels were reconstructed from within the aneurysm by directly anastomosing openings in the main Dacron graft to the aortic origin of these vessels. Since graft replacement of TAAA implies at least temporary interruption of visceral and potentially spinal cord blood flow, operative management of these lesions may be complicated by ischemic damage in these vascular beds. Despite various surgical and adjunctive strategies applied in different centers to minimize overall operative morbidity, the state of the art in contemporary management of these patients still entails a 5% to 10% risk of perioperative mortality and/or morbidity in the form of renal, respiratory, and spinal cord ischemic complications (SCI). Efforts to supplant conventional open surgery with totally endovascular repair are as yet in their infancy, but can be anticipated to play an ever-increasing role.
Etiology
The majority of TAAAs are degenerative in nature and occur in association with hypertension, smoking, and frequently with evidence of vascular disease in other territories. Up to 20% of TAAAs in most series are the sequelae of chronic aortic dissection; such patients are typically younger and may be afflicted with syndromic conditions such as Marfan’s syndrome. The male:female sex ratio of TAAAs in our patients is 1:1, whereas our experience with AAA patients is consistent with the 5 to 6:1 male:female sex ratio frequently reported in the literature. Others have noted the tendency for aneurysm disease in females to more often involve proximal aortic segments. Uncommon causes of TAAA include lesions that result from infection in a preexistent degenerative aneurysm or more commonly false aneurysm formation after infectious aortitis. Finally, a variety of congenital abnormalities, such as neurofibromatosis and/or the spectrum of vasculitis/aortitis syndromes can lead to TAAA; these typically constitute but 1% to 2% of cases.
Classification and Natural History
Anatomy
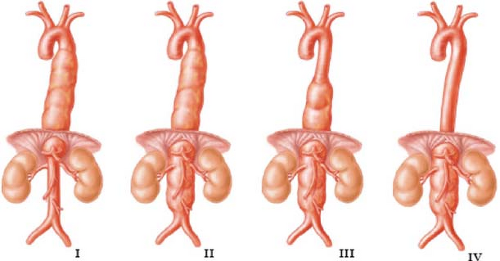
TAAAs are classified according to the scheme originally devised by Crawford, which in the most basic terms considers whether the lesion is primarily a caudal extension of a descending thoracic aneurysm or a cephalad extension of a total abdominal aneurysm (Fig. 1). This classification is clinically useful since it has direct implications for both the technical conduct of operation and the risk of perioperative complications, in particular SCI. There is considerable variation in the overall scope of operation required to deal with lesions within the classification of TAAA. In contemporary practice, management of the Type IV TAAA should be accomplished with an overall morbidity and mortality not significantly different from the management of routine AAAs, and indeed this has been the case in our practice. It is clear that the same cannot be said for the more extensive Type I and Type II lesions. We reserve the designation Type II aneurysm for those patients where the entire descending thoracic aorta is involved. Repair of these aneurysms typically requires a clamp placed directly at or even proximal to the left subclavian artery. Furthermore, the aneurysm should be classified according to the extent of aorta resected during a single procedure. For example, it is commonplace to resect a Type I aneurysm down to a prior infrarenal aneurysm repair. Such lesions should be classified as a Type I rather than a Type II aneurysm. These considerations explain the considerable variation reported in the literature with respect to distribution of TAAA extent. While the degree of variability is evident, some 50% to 60% of treated aneurysms fall into the more extensive Type I and Type II lesions.
The expected natural history of TAAAs is progressive enlargement and eventual rupture. Population-based studies place the incidence of TAAAs at 5 to 6 per 100,000 people with an actuarial 5-year survival as low as 13%. This is consistent with single center studies that report less than a quarter of patients referred for surgical treatment but subsequently denied treatment were alive 2 years later. As in the case of studies detailing the natural history of AAAs, increasing aneurysm diameter, an increase in the rate of expansion, the presence of chronic obstructive pulmonary disease (COPD), steroid use, female sex, advanced age, and the presence of renal insufficiency
all predict a higher risk of rupture. As the risk of rupture for aneurysms less than 5 cm is negligible, we have adopted 6 cm as an appropriate threshold to recommend surgical intervention for TAAAs of degenerative or chronic dissection etiology after comorbid conditions and age are considered. For TAAAs secondary to chronic dissection in young patients and/or those with those with Marfan’s syndrome, a 5-cm threshold is used.
all predict a higher risk of rupture. As the risk of rupture for aneurysms less than 5 cm is negligible, we have adopted 6 cm as an appropriate threshold to recommend surgical intervention for TAAAs of degenerative or chronic dissection etiology after comorbid conditions and age are considered. For TAAAs secondary to chronic dissection in young patients and/or those with those with Marfan’s syndrome, a 5-cm threshold is used.
Clinical Presentation
Presentation in totally asymptomatic fashion is common, as is detection of TAAAs during radiographic investigations carried out for other causes. Alternatively, symptoms referable to the TAAAs are often seen. Acutely developing, severe pain may be associated with aneurysm expansion, rupture, and/or acute dissection. Large TAAAs may produce symptoms of chronic back, epigastric, or flank pain presumably related to local compressive phenomenon. When severe, this can represent a chronic state of contained aneurysm rupture. New onset of hoarseness related to left recurrent laryngeal nerve palsy, compression or erosion of the tracheobronchial tree, pulmonary parenchyma producing cough, hemoptysis, or dyspnea, and dysphasia lusoria are possible, but uncommon. Perhaps related to reluctance to recommend operation because of the threat of surgical morbidity, up to 40% of TAAA patients will present with prior symptoms. Our results are consistent with those available from a review of the literature indicating that some 25% of patients will be treated in urgent or emergent circumstances, with approximately half of these treated for frank rupture.
Imaging
Accurate and complete radiographic evaluation is essential for precise operative planning with no equivocation in the surgeon’s mind as to the proximal and distal extent of aortic resection. A fine-cut, contrast-enhanced computerized tomography (CT) scan with helical reconstruction provides the surgeon with (a) the proposed location of and a qualitative assessment of the aorta in the region of the proximal cross-clamp, (b) the assessment of patency of the visceral vessels, (c) the topography and location of the renal artery origins, in addition to kidney size and adequacy of perfusion, (d) the distal extent of the resection, (e) the aneurysmal status of the iliac vessels, as well as possible occlusive disease in the pelvis in case the operation is to be performed with retrograde transfemoral aortic perfusion. All iodinated contrast diagnostic studies are performed well in advance of actual surgery. The CT scan is also the initial diagnostic test of choice in patients presenting with acute pain and/or where the possibility of aneurysm rupture exists. If indeed rupture is documented, no further studies are indicated and prompt operation is carried out.
Since the majority of patients seen for TAAA resection are those with degenerative aneurysms, demographic and clinical features typical of a patient population with diffuse atherosclerosis are the rule. Patients treated for degenerative aneurysm average 70 years in age and a history of hypertension is nearly universal. Cigarette smoking and/or significantCOPD is common, with 25% of patients having significant COPD as manifested by an FEV1 of less than 50% predicted. Cerebrovascular disease, prior stroke, and symptomatic manifestations of lower extremity arterial occlusive disease occur in 15% of patients. Associated visceral and/or renovascular occlusive disease occur to some degree in 30% of patients. Such lesions, of course, have direct implications for the technical conduct of the operation and the incidence and significance of chronic renal insufficiency. These patients are more likely to be older and to harbor some degree of renal function compromise; their overall incidence of complications after operation, in particular, renal failure, was significantly increased when compared with patients without visceral artery occlusive disease. The coexistence of renovascular disease and some degree of renal insufficiency is commonplace in TAAA patients and has important implications for accurate
assessment of perioperative risk and long-term preservation of renal function. With the exception of Type I TAAA, which frequently will terminate just proximal to the renal artery level, types II, III, and IV thoracoabdominal aneurysm designation implies aneurysmal degeneration of the entire visceral aortic segment. Some patients with renal insufficiency will have the potential for retrieval or salvage of renal function with renal artery reconstruction. We believe extreme levels of preoperative azotemia (serum creatinine greater than 2.5 cm/dL) constitute a relative contraindication to elective operation unless preoperative studies indicate some potential for salvage or retrieval of renal function with renal artery reconstruction.
assessment of perioperative risk and long-term preservation of renal function. With the exception of Type I TAAA, which frequently will terminate just proximal to the renal artery level, types II, III, and IV thoracoabdominal aneurysm designation implies aneurysmal degeneration of the entire visceral aortic segment. Some patients with renal insufficiency will have the potential for retrieval or salvage of renal function with renal artery reconstruction. We believe extreme levels of preoperative azotemia (serum creatinine greater than 2.5 cm/dL) constitute a relative contraindication to elective operation unless preoperative studies indicate some potential for salvage or retrieval of renal function with renal artery reconstruction.
Cardiac and Pulmonary Risk Stratification
Irrespective of the firm literature base against routine preoperative cardiac testing, all patients should be evaluated with physiologic testing to assess perioperative myocardial ischemic potential. In addition, patients with a history or symptoms suggestive of heart failure should have an assessment of left ventricular function. While patients with significant impairments of pulmonary reserve can usually be detected on a historical basis alone, we routinely obtain preoperative pulmonary function studies. Preoperative consultation with a pulmonologist for optimization of bronchodilator therapy and pulmonary toilet is an important component in the management of patients with significant COPD. However, institution of preoperative steroid therapy with the intent of improving respiratory function is contraindicated since we have observed this maneuver to precipitate aneurysm rupture. Advanced age is an important component only in as much as it is accompanied by overall fragility and impaired functional status.
General Principles of Operation
Graft replacement by a direct surgical approach is the current standard treatment for TAAA. Total endovascular repair has been plagued by device and regulatory constraints and is not generally available; hybrid operation has perhaps increased the pool of surgeons treating TAAA, but the reported results, including our own, compare poorly with conventional operation in competent centers. Nonoperative therapy may be selected initially in very elderly patients, those with modest-size aneurysms (see above), and those for whom associated comorbid conditions make the short-term risk of surgery prohibitive and/or life expectancy limited to a degree that surgical treatment is not rational. Patients selected for nonoperative therapy should be treated aggressively with beta-blockade, hypertension control, and cessation of cigarette smoking. Major clinical series emphasize a significant incidence of prior aortic resections (1/3 of patients in our series); the most common pattern is the patient who has undergone a prior infrarenal AAA repair (60% of total prior resections). While synchronous proximal aneurysm is noted in 6% to 13% of degenerative TAAA patients, contiguous arch aneurysm is rare, typically occurring only in patients with a prior DeBakey Type I aortic dissection, which requires complex, staged repairs. Dr. Crawford’s emphasis on an expeditious operation, minimizing cross-clamp time, heparin use, and blood turnover are principles we continue to apply with certain modifications.
Protective Adjuncts and Motor-Evoked Potential Monitoring
Renal and Spinal Cord Protection
Outcomes in TAAA repair are closely correlated with renal and spinal cord complications; accordingly, operative adjuncts to minimize these complications have been principal drivers of the technical conduct of the operation. Consistent with a firm literature base supporting regional hypothermic protection of the kidneys, our approach involves direct installation of renal preservation fluid (Lactated Ringers with 25 g of mannitol/L and 1 g/L methyl prednisolone at 4°C) into the renal artery ostia after the aorta is opened. Initially, 250 mL of this solution is instilled into each renal artery ostium and a continuous drip of the same begun through size 6 French perfusion balloon-tipped catheters. Experience has shown that such an infusion will result in a rapid decline of renal parenchymal temperature to 15°C after the bolus infusion. During the continuous infusion, renal core temperatures remain in the 25°C level as monitored by direct temperature probes in the renal cortex.
The general topic of adjuncts for spinal cord protection is beyond the scope of this chapter and the reader is referred to collective reviews on the topic. Such adjuncts can be broadly divided into those that preserve spinal cord blood flow such as cerebrospinal fluid drainage (CSFD), distal aortic perfusion, and intercostal reconstruction, and, second, neuroprotective adjuncts that in clinical practice largely involve variations on the theme of hypothermia. There exists a firm literature base supportive of CSFD for spinal cord protection; the same cannot be said of the multiple other adjuncts currently in use. We previously described and applied a technique of regional cord hypothermia and demonstrated that it produced a significant reduction in SCI. Nonetheless, SCI could not be reduced beyond a finite approximate 8% level. These data coupled with the development of the collateral network concept referable to spinal cord blood flow, led to a transition in our operative approach wherein epidural cooling yielded to distal aortic perfusion with motor-evoked potential (MEVOP) monitoring (coupled with CSFD) as the principal cord protective strategy. Elegant magnetic resonance imaging studies of spinal cord circulation combined with intraoperative MEVOP data reported by Jacobs et al. indicated that (a) individual intercostal vessels were typically not “critical” for cord preservation and (b) most collaterals that support the cord originated distal to the distal clamp (i.e., the pelvis), and preservation of continuous perfusion thereof accordingly was logical. Finally, this approach allows for selective (based on intraoperative MEVOP) rather than routine intercostal reconstruction. MEVOP monitoring does mandate a departure from anesthetic techniques typically used in North America, and the technical requirements of such monitoring can only be satisfied by a dedicated team specialized in neurophysiology. Online intraoperative consultation is often required to interpret potential deterioration of potentials.
Technical Facets of Reconstruction
Operative Exposure
Irrespective of individual preferences about the various technical components of the operation, the absolute requirement for a successful technical operation is the provision of broad continuous exposure of the entire left posterolateral aspect of the aorta. Particularly in extensive Type II aneurysm, maneuvers that tend to improve proximal exposure can compromise distal exposure and vice versa. The location and extent of the thoracic portion of the incision is dictated by the
proximal extent of the aneurysm. The posterior portion of a standard posterolateral thoracotomy is only necessary for Type I and Type II aneurysms. We keep the thoracic portion of the incision low and have found that the fifth or sixth interspace incisions provide adequate exposure for the majority of even the more proximal aneurysms. The costal margin is divided at the level of the sixth interspace and a self-retaining retractor system is essential to have continuous exposure of the entire operative field. An eighth interspace thoracoabdominal incision will usually suffice for type IV aneurysms, and a double-lumen tube for deflation of the left lung is generally not necessary in these cases. We prefer to keep the abdominal portion of the incision well lateral on the abdominal wall rather than extending to the midline. This has the advantage of allowing the visceral contents to lie within the abdominal cavity and of decreasing evaporative fluid and heat losses. The abdominal portion of the incision is transperitoneal as this allows for direct inspection and assessment to the visceral circulation at the conclusion of operation.
proximal extent of the aneurysm. The posterior portion of a standard posterolateral thoracotomy is only necessary for Type I and Type II aneurysms. We keep the thoracic portion of the incision low and have found that the fifth or sixth interspace incisions provide adequate exposure for the majority of even the more proximal aneurysms. The costal margin is divided at the level of the sixth interspace and a self-retaining retractor system is essential to have continuous exposure of the entire operative field. An eighth interspace thoracoabdominal incision will usually suffice for type IV aneurysms, and a double-lumen tube for deflation of the left lung is generally not necessary in these cases. We prefer to keep the abdominal portion of the incision well lateral on the abdominal wall rather than extending to the midline. This has the advantage of allowing the visceral contents to lie within the abdominal cavity and of decreasing evaporative fluid and heat losses. The abdominal portion of the incision is transperitoneal as this allows for direct inspection and assessment to the visceral circulation at the conclusion of operation.
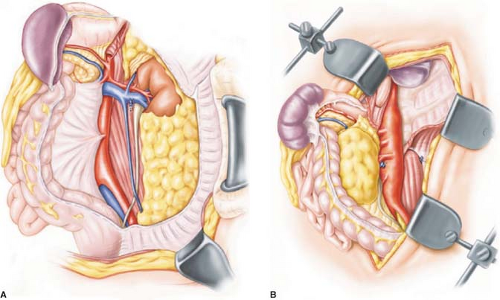
Exposure of the left posterolateral aspect of the abdominal aorta is obtained by entering the plane posterior to the spleen, left kidney, and left colon with the electrocautery. All intrabdominal contents are reflected to the patient’s right side and the left ureter is identified and protected (Fig. 2). In addition to the retroperitoneal fatty and lymphatic tissues, the renal–lumbar vein courses across the aorta and typically is a convenient guide to the left renal artery. A key point in the dissection is identifying the renal artery and dissecting it back to its aortic origin. This is in turn the starting point for cephalad and caudad division of the retroperitoneal tissues and division of the median arcuate ligament and diaphragmatic crura superiorly.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree