Thoracic Aortic Transection
Himanshu J. Patel
G. Michael Deeb
Blunt thoracic aortic injury (BTAI) is the second leading cause of death from vehicular trauma. The classic autopsy study by Parmley et al. as well as more contemporary studies have suggested that the mortality of BTAI exceeds 85% at the time of injury, while mortality risk beyond 4 hours following presentation remains low at 6%. More recently, selective delayed operative management advocated by our group as well as others suggests that with aggressive antihypertensive therapy, survival rates following admission are higher than those seen in autopsy reports.
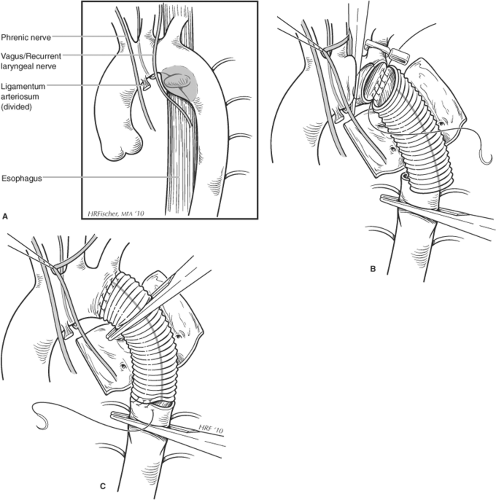
Although the location of injury can occur at any site in the aorta, the most frequent location identified in operative series is the aortic segment just distal to the ligamentum arteriosum. Although the pathogenesis remains debated, potential etiologies for disruption include both differential deceleration forces on the aorta relative to surrounding tissues and thoracic compression forces impacting the aorta directly.
BTAI can have a spectrum of injury ranging from complete disruption with a true double-barrel aorta to a partial wall disruption with pseudoaneurysm formation and finally to an isolated intimal injury without associated mural hematoma. This spectrum of disease likely results in disparate trajectories in the natural history. Isolated intimal injury is likely a benign process, and will often heal spontaneously without intervention. Partial wall disruption will often result in pseudoaneurysm formation medially into the mediastinum with associated mediastinal hematoma, and on exploration resembles an entry tear of an acute type B dissection. Those presenting with lateral wall (i.e., projecting into lung parenchyma) involvement are considered by us to be somewhat more unstable, and are less frequent perhaps because these patients have succumbed to free rupture. Finally, complete disruption is considered to be the least stable pathology and at the highest risk for rupture. In general, tears in the partial and complete disruption transections are often transverse in orientation, and the natural longitudinal tension in the aorta separates the disruption by several centimeters.
BTAI should be suspected on initial primary survey, based upon both the mechanism of injury and physical signs or imaging findings. High-impact mechanism including vehicular accidents with significant deceleration forces, falls, or blast injuries should prompt evaluation for BTAI. Physical signs, such as first or second rib or thoracic spine fractures, shock, dyspnea, or hemothorax, are also suggestive.
Imaging studies are of vital importance in confirming the diagnosis of BTAI. Aortography, widely considered the gold standard in evaluation for BTAI, has a near 100% sensitivity and specificity in experienced hands, but is time-consuming and often negative. An often underutilized tool in diagnosis is the transesophageal echocardiogram (TEE). The main advantage of this test is its portability, and its use can be important in those patients presenting with a requirement for immediate surgery for concomitant injuries. At the time of operative repair of concomitant injuries, TEE can be performed safely during the operative procedure to obtain the diagnosis. The previously routine use of aortography in diagnosis of BTAI has now been supplanted by the widespread use of computed tomography (CT), which in the modern era is obtained rapidly and can also be used in aiding diagnosis of concomitant injuries. Suggestive findings on CT scanning include mural and mediastinal hematoma, intimal flaps, or pseudoaneurysm formation. This test is important for multiple reasons, particularly in the endovascular era where endograft sizing is dependent on high-quality thin cut (1.25 to 3 mm) imaging.
In BTAI, the indication for operation is aortic repair for improvement in life expectancy. As discussed earlier, we believe that not all aortic injuries require operation. While the partial and complete disruptions have a risk for rupture without repair, intimal injuries or mural hematomas likely do not portend the same risk. We and others have noted that a large percentage of patients will spontaneously heal these lesions with aggressive medical therapy. In our experience, these do not subsequently develop pseudoaneurysms without surgery and likely have a relatively low risk for rupture.
Following presentation, patients are immediately treated with parenteral antihypertensive therapy. At our institution, we will aggressively reduce blood pressure for at least a week (goal systolic BP 100 to 120 mm Hg), with beta blockers used as a first-line agent. If repair is delayed by more than 1 week for reasons of comorbidities or associated injuries, we then liberalize control to let systolic pressures rise to no more than 150 mm Hg, and institute oral pharmacotherapy. If operative repair is delayed, we will obtain repeat imaging at 48 hours to be certain that there is no significant change in the injury, such as rapid growth of pseudoaneurysm or increased mediastinal hematoma. It is not unusual for patients to develop pleural
effusions that can increase in size over the first 24 to 48 hours with volume resuscitation, and this in and of itself does not represent an indication for more urgent repair.
effusions that can increase in size over the first 24 to 48 hours with volume resuscitation, and this in and of itself does not represent an indication for more urgent repair.
Repair of the aortic injury is performed as soon as appropriate. However, life-threatening abdominal or neurologic injuries will preclude early repair. In our experience, the most frequent reason for operative delay involves the presence of closed head injury precluding use of heparin for either open or endovascular repair. Another frequent reason is the presence of significant pulmonary contusion, precluding use of single lung ventilation for open descending aortic repair (DTAR) (Fig. 1). In the current era, if endovascular repair (i.e., thoracic aortic endovascular repair (TEVAR)) is not felt appropriate, we will delay the open procedure until the time when the patient’s pulmonary status allows proceeding with surgery (Fig. 2). Finally, in the event that deep hypothermic circulatory arrest is deemed a necessary adjunct (<5%), we will often wait until the patients have been extubated, and have undergone some degree of rehabilitation, as this operative approach requires a significantly increased physiologic reserve.
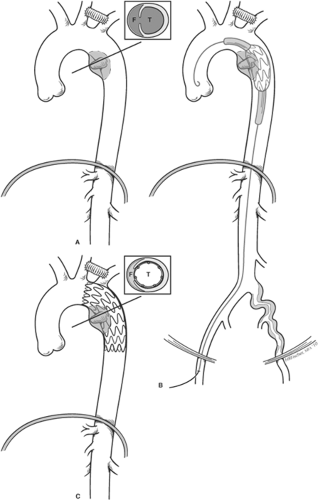
The determination of type of repair (i.e., DTAR vs. TEVAR) is based upon several premises. Unlike some institutions that espouse one or another approach exclusively, we take a more conservative strategy understanding that we have an extensive positive experience with both open and endovascular approaches to the descending aorta. There are no long-term data (10 to 20 years) reported with TEVAR for BTAI, and this is an important consideration in the younger population. Another important consideration is that frequently follow-up is incomplete, making serial imaging that is needed after TEVAR impossible in all patients. A growing concern is also that the serial imaging needed after TEVAR is associated with a large accumulated contrast and radiation exposure over the patient’s lifespan, particularly in the younger population. Finally, the configuration of the arch is more hazardous
in the younger patient population, particularly with the currently available stent graft technology.
in the younger patient population, particularly with the currently available stent graft technology.
In incorporating this information, we regard the gold standard therapy as an open approach for BTAI. Certain patient populations, such as older patients, may benefit from an endovascular approach, both because of the impact of open repair on early recovery, morbidity and mortality, and because the aortic arch anatomy may be more conducive for TEVAR. We will preferentially use TEVAR in patients over 60 years of age, and in those who have significant or incompletely evaluated comorbid conditions, such as coronary artery disease without known extent, congestive heart failure, chronic renal failure, or emphysema. If TEVAR is not felt suitable, we will then delay the open repair if possible until these conditions have been more adequately evaluated.
Conventional Open Repair
Preoperative Planning
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree