Therapeutic drug monitoring
When is therapeutic drug monitoring required?
For many drugs, the dose given correlates well with a pharmacological effect, and the correct dosage can be satisfactorily determined by clinical assessment or the measurement of a biochemical response. For example, the pharmacological actions of anti-coagulants and of anti-hypertensive drugs can be assessed, and dosage adjusted, on the basis of prothrombin time and blood pressure measurements, respectively.
Measurement of plasma or blood drug levels is required:
Table 20.1 lists the drugs for which the case for therapeutic drug monitoring (TDM) has been clearly established. Regular monitoring of patients taking these drugs is not usually required once the patient has been stabilised on a dose of drug that has produced the desired clinical effect.
Table 20.1 Therapeutic drug monitoring; examples of drugs for which it is indicated

TDM is important if there is a particular clinical problem to be addressed, such as:
When should the blood sample be taken?
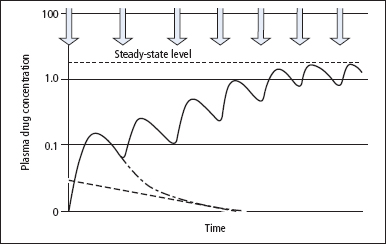
When a single dose of drug is taken for the first time, plasma levels will initially rise rapidly and then decline in a curvilinear manner similar to that shown in Figure 20.1. The characteristics of this curve provide essential details about the kinetics of the drug and the information needed to calculate the approximate dose and frequency of the drug for the desired therapeutic concentration in plasma. For any drug given at regular intervals, a steady-state relatively constant concentration in plasma is reached after about five half-lives (Figure 20.1). However, peak levels (achieved just after administration) and trough levels (achieved immediately prior to the next dose) may still be recognised. For most drugs, it is important that trough levels are adequate to achieve the desired therapeutic effect. Thus, blood samples are often withdrawn just before a dose of the drug is taken, but at any rate samples should usually be taken after the initial peak has subsided, unless toxicity is suspected.
In all cases, the time of blood sampling and of the last dose of the drug must be given.
Figure 20.1 The effect on plasma drug concentration of giving repeated regular doses of a drug. As can be seen, after approximately five doses of the drug, a steady state-level is reached, with peak and trough values being found. The hatched line represents the elimination half-life and the hatched/dotted line shows the plasma drug concentration profile if a single dose of the drug had been given. Arrows show the time of each dose of the drug.
Interpretation of drug levels
Therapeutic ranges If the blood has been taken at the appropriate time, the plasma level can be compared with published therapeutic ranges (Table 20.1). These published ranges indicate the range of plasma drug levels which in the majority of the population have been shown to provide the desired therapeutic effect without a high risk of toxicity. Published ranges offer little more than guidelines, because of inter-individual variation in the clinical response to drugs.
Interpretation of a result requires:
Other important issues in therapeutic drug monitoring
Free and bound drugs
Most drugs circulate partly bound to plasma proteins, the bound and unbound forms being in equilibrium. The pharmacological response is usually determined by the tissue concentration which, in turn, is related to the plasma [unbound drug]. However, plasma [free drug] is difficult to measure, and TDM depends on the measurements of plasma [total drug]. For some drugs, the concentration of drug in saliva may reflect the concentration of free drug in plasma, and salivary drug measurements are now widely used in screening for drugs of abuse.
The importance of drug metabolites
Most drugs are metabolised to inactive products, although some are inactive when taken and are converted to active drug in the liver or GI tract. For example, primidone is converted to active metabolites, principally phenobarbitone. If primidone therapy is to be monitored, plasma [phenobarbitone] is the measurement required.
A number of different analytical methods are sometimes used for measurement of a single drug (and possibly of its metabolites). This explains the differences that may be found between results from different laboratories. It also explains why some less specific methods may give rise to misleading results if there are high concentrations of inactive metabolites in plasma. Even specific methods may give rise to misleading results if they fail to measure active metabolites.
Units of measurement
Plasma drug concentrations may be expressed in mass (gravimetric) or molar units of concentration (Table 20.1). The practice of referring to numerical values of drug measurements without mentioning the units is dangerous, since it can lead to serious – and sometimes fatal – mistakes.
Specific drugs
Aminoglycoside antibiotics
These antibiotics have a very short half-life of 2–3 h if renal function is normal, but in patients with infection or renal impairment the half-life becomes prolonged (up to 100 h). In addition, tissue pools of gentamycin may become saturated if treatment is for more than a week, and then plasma levels may start to rise sharply. Gentamycin is nephrotoxic and ototoxic. Therefore, TDM is particularly important in patients with impaired renal function who receive the drug for more than 7 days, or those on high loading doses for serious infection. Peak and trough levels should be measured.
Anti-convulsants
Carbamazepine Carbamazepine has fewer side effects, mainly neurotoxic, than phenytoin. Monitoring is of value in patients with poor control, since there is a variable relationship between dose and plasma concentration. The sample should be taken just before a dose.
Phenytoin Phenytoin has a low therapeutic ratio and is subject to variable rates of hepatic metabolism, leading to a non-linear relationship between dose and plasma concentration. Because of its undesirable side effects, which include neurotoxicity and increased frequency of fits, TDM is required in new patients, where there is an unexpected loss in control, in pregnancy or when other drugs that interact with phenytoin are added or withdrawn.
Drugs that prevent graft rejection
A number of drugs are used to prevent graft rejection. TDM is recommended to ensure efficacy of each of these drugs since achieving the correct level of drug in the blood is essential to prevent rejection while minimising side effects such as nephrotoxicity. Some centres may use combinations of these drugs.
Ciclosporin is nephrotoxic, with signs that may mimic rejection in patients with transplants. The peak level (achieved 2h after the dose, known as the C2 level) provides a better means of guiding dosage than the trough level.
Tacrolimus and sirolimus are relatively new immunosuppressive drugs that may have lower toxicity than ciclosporin. Therapeutic monitoring of blood levels of these drugs is still required.
Digoxin
Digoxin has little clinical effect at plasma concentrations below 1 nmol/L, whereas toxicity (often manifest as cardiac arrhythmia and vomiting) is common when plasma levels rise above 3.8 nmol/L. Digoxin results should always be interpreted together with a plasma potassium concentration, since hypokalaemia potentiates the effect of digoxin. Thus, toxic effects of the drug may occur in a hypokalaemic patient who has a plasma digoxin within the therapeutic range. A similar effect may be seen in hypercalcaemia, hypomagnesaemia and hypothyroidism. Equilibration of digoxin with cardiac tissue takes some time, and thus blood should not be taken for at least 6h after the dose.
Lithium
Lithium is used for the treatment of depressive illness. It has a short half-life, and plasma levels should be determined 12 h after the last dose. TDM is essential because the drug is toxic, producing a range of symptoms including polyuria, hypothyroidism and, in severe cases, renal failure and coma. Patients with plasma [lithium] above 1.4 mmol/L are at risk of oliguria and acute renal failure. TDM may also be necessary in order to monitor compliance.
Methotrexate
Methotrexate is a dihydrofolate reductase inhibitor, and therefore reduces intracellular folate, which in turn inhibits DNA synthesis. The drug is cytotoxic; high-dose regimens are used in the treatment of some cancers, and lower dose regimens are used for immunosuppression. TDM is of value in patients receiving high doses of methotrexate, to identify those at risk of toxic effects and to provide a guide to the dose and timing of leucovorin (a drug that restores the pool of reduced folate) rescue.
Theophylline
This drug is used to prevent or treat bronchocon-striction in some children or elderly patients who cannot use an inhaler easily. The drug commonly produces minor side effects such as nausea and headache, even at concentrations within the therapeutic range. Serious toxicity leading to cardiac arrhythmia can occur with plasma levels above 110 mmol/L. TDM is particularly valuable to optimise the dose, confirm toxicity or demonstrate poor compliance. Some believe that the metabolite caffeine should also be measured.
Pharmacogenomics
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


