INTRODUCTION
The staphylococci are gram-positive spherical cells, usually arranged in grapelike irregular clusters. They grow readily on many types of media and are active metabolically, fermenting carbohydrates and producing pigments that vary from white to deep yellow. Some are members of the normal microbiota of the skin and mucous membranes of humans; others cause suppuration, abscess formation, a variety of pyogenic infections, and even fatal septicemia. The pathogenic staphylococci often hemolyze blood, coagulate plasma, and produce a variety of extracellular enzymes and toxins. The most common type of food poisoning is caused by a heat-stable staphylococcal enterotoxin. Staphylococci rapidly develop resistance to many antimicrobial agents, which consequently presents difficult therapeutic problems.
The genus Staphylococcus has at least 45 species. The four most frequently encountered species of clinical importance are Staphylococcus aureus, Staphylococcus epidermidis, Staphylococcus lugdunensis, and Staphylococcus saprophyticus. S aureus is coagulase positive, which differentiates it from the other species. S aureus is a major pathogen for humans. Almost every person will have some type of S aureus infection during a lifetime, ranging in severity from food poisoning or minor skin infections to severe life-threatening infections. The coagulase-negative staphylococci (CoNS) are normal human microbiota and sometimes cause infection, often associated with implanted devices, such as joint prostheses, shunts, and intravascular catheters, especially in very young, old, and immunocompromised patients. Approximately 75% of these infections caused by coagulase-negative staphylococci are caused by S epidermidis; infections caused by S lugdunensis, Staphylococcus warneri, Staphylococcus hominis, and other species are less common. S saprophyticus is a relatively common cause of urinary tract infections in young women, although it rarely causes infections in hospitalized patients. Other species are important in veterinary medicine.
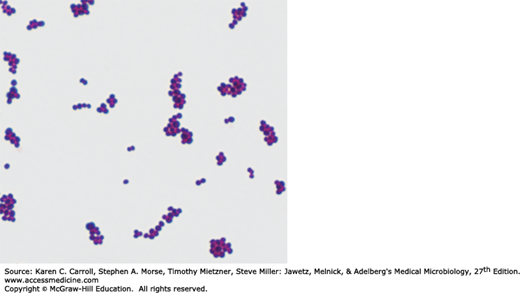
Staphylococci are spherical cells about 1 μm in diameter arranged in irregular clusters (Figure 13-1). Single cocci, pairs, tetrads, and chains are also seen in liquid cultures. Young cocci stain strongly gram positive; on aging, many cells become gram negative. Staphylococci are nonmotile and do not form spores. Under the influence of drugs such as penicillin, staphylococci are lysed.
Micrococcus species often resemble staphylococci. They are found free living in the environment and form regular packets of four (tetrads) or eight cocci. Their colonies can be yellow, red, or orange. Micrococci are rarely associated with disease.
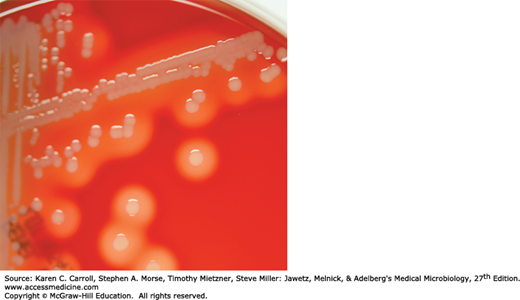
Staphylococci grow readily on most bacteriologic media under aerobic or microaerophilic conditions. They grow most rapidly at 37°C but form pigment best at room temperature (20–25°C). Colonies on solid media are round, smooth, raised, and glistening (Figure 13-2). S aureus usually forms gray to deep golden yellow colonies. S epidermidis colonies usually are gray to white on primary isolation; many colonies develop pigment only upon prolonged incubation. No pigment is produced anaerobically or in broth. Various degrees of hemolysis are produced by S aureus and occasionally by other species. Peptostreptococcus and Peptoniphilus species, which are anaerobic cocci, often resemble staphylococci in morphology. The genus Staphylococcus contains two species, Staphylococcus saccharolyticus and S aureus subsp. anaerobius, which initially grow only under anaerobic conditions but become more aerotolerant on subcultures. This may be seen on rare occasions with some strains of S epidermidis as well.
The staphylococci produce catalase, which differentiates them from the streptococci. Staphylococci slowly ferment many carbohydrates, producing lactic acid but not gas. Proteolytic activity varies greatly from one strain to another. Pathogenic staphylococci produce many extracellular substances, which are discussed below.
Staphylococci are relatively resistant to drying, heat (they withstand 50°C for 30 minutes), and 10% sodium chloride but are readily inhibited by certain chemicals (eg, 3% hexachlorophene).
Staphylococci are variably susceptible to many antimicrobial drugs. Resistance is caused by several mechanisms:
β-Lactamase production is common, is under plasmid control, and makes the organisms resistant to many penicillins (penicillin G, ampicillin, ticarcillin, piperacillin, and similar drugs). The plasmids are transmitted by transduction and perhaps also by conjugation.
Resistance to nafcillin (and to methicillin and oxacillin) is independent of β-lactamase production. Resistance to nafcillin is encoded and regulated by a sequence of genes found in a region of the chromosome called the staphylococcal cassette chromosome mec (SCCmec). Specifically, the mecA and newly described mecC genes on this locus encode a low-affinity penicillin-binding protein (PBP2a) that is responsible for the resistance. There are 12 different SCCmec types. Types I, II, III, VI, and VIII are associated with hospital-acquired infections (HA-MRSA) and may contain genes that encode resistance to other antimicrobials as well. SCCmec type IV has principally been found in community-acquired methicillin-resistant S aureus (CA-MRSA) strains that tend to be less resistant, more transmissible, and responsible for outbreaks over the past decade in the United States and some countries in Europe. Types IX and X are associated with animals (livestock-associated MRSA [LA-MRSA]) of which type IX contains mecC. The other types have been limited to various geographic locations around the world.
In the United States, S aureus and S lugdunensis are considered to be susceptible to vancomycin if the minimum inhibitory concentration (MIC) is 2 μg/mL or less; of intermediate susceptibility if the MIC is 4–8 μg/mL; and resistant if the MIC is 16 μg/mL or greater. Strains of S aureus with intermediate susceptibility to vancomycin have been isolated in Japan, the United States, and several other countries. These are often known as vancomycin-intermediate S aureus (VISA). They generally have been isolated from patients with complex infections who have received prolonged vancomycin therapy. Often there has been vancomycin treatment failure. The mechanism of resistance is associated with increased cell wall synthesis and alterations in the cell wall and is not caused by the van genes found in enterococci. S aureus strains of intermediate susceptibility to vancomycin usually are nafcillin resistant but generally are susceptible to oxazolidinones and to quinupristin–dalfopristin.
Since 2002, several isolates of vancomycin-resistant S aureus (VRSA) strains (MICs ≥ 16 μg/mL) were isolated from patients in the United States. The isolates contained the vancomycin resistance gene vanA likely derived from enterococci (see Chapter 14) and the nafcillin resistance gene mecA (see above). Both of the initial VRSA strains were susceptible to other antibiotics. Vancomycin resistance in S aureus is of major concern worldwide.
Plasmid-mediated resistance to tetracyclines, erythromycins, aminoglycosides, and other drugs is frequent in staphylococci.
“Tolerance” implies that staphylococci are inhibited by a drug but not killed by it—that is, there is great difference between minimal inhibitory and minimal lethal concentrations of an antimicrobial drug. Patients with endocarditis caused by a tolerant S aureus may have a prolonged clinical course compared with patients who have endocarditis caused by a fully susceptible S aureus. Tolerance can at times be attributed to lack of activation of autolytic enzymes in the cell wall.
A culture of staphylococci contains some bacteria that differ from the bulk of the population in expression of colony characteristics (colony size, pigment, hemolysis), in enzyme elaboration, in drug resistance, and in pathogenicity. In vitro, the expression of such characteristics is influenced by growth conditions: When nafcillin-resistant S aureus is incubated at 37°C on blood agar, one in 107 organisms expresses nafcillin resistance; when it is incubated at 30°C on agar containing 2–5% sodium chloride, one in 103 organisms expresses nafcillin resistance. Some isolates may develop alterations in phenotypes such as smaller size (pin point colonies) and loss of hemolysis. These are referred to as small colony variants (SCVs) and the variations in phenotypic characteristics enable better survival under intracellular conditions, facilitating persistence and leading to chronic infections.
S aureus has amazing adaptive capacity. Full genome sequencing of numerous isolates (www.ncbi.nlm.nih.gov/genome/genomes/154) has elucidated the evolution of various structures, toxins, and enzymes that this organism has developed over time. S aureus has acquired many mobile genetic elements (eg, insertion sequences, transposons, etc) that determine both pathogenicity and antimicrobial resistance (see Regulation of Virulence Determinants).
Staphylococci contain antigenic polysaccharides and proteins as well as other substances important in cell wall structure. Peptidoglycan, a thick polysaccharide polymer containing linked subunits, provides the rigid exoskeleton of the cell wall and anchors the adhesins (see below). Peptidoglycan is destroyed by strong acid or exposure to lysozyme. It is important in the pathogenesis of infection: It elicits production of interleukin-1 (endogenous pyrogen) and opsonic antibodies by monocytes, and it can be a chemoattractant for polymorphonuclear leukocytes, have endotoxin-like activity, and activate complement. Peptidoglycan assembly is a target of β-lactam and glycopeptide antimicrobial agents.
Teichoic acids, which are polymers of polyribitol–phosphate, are cross-linked to the peptidoglycan and can be antigenic. They are important in cell wall metabolism. Antiteichoic acid antibodies detectable by gel diffusion may be found in patients with active endocarditis caused by S aureus.
Protein A is a cell wall component of S aureus strains and is a bacterial surface protein that has been characterized among a group of adhesins called microbial surface components recognizing adhesive matrix molecules (MSCRAMMs). Bacterial attachment to host cells is mediated by MSCRAMMs, and these are important virulence factors. Protein A binds to the Fc portion of IgG molecules except IgG3. The Fab portion of the IgG bound to protein A is free to combine with a specific antigen. Protein A has become an important reagent in immunology and diagnostic laboratory technology; for example, protein A with attached IgG molecules directed against a specific bacterial antigen agglutinates bacteria that have that antigen (“coagglutination”). Another important MSCRAMM is clumping factor on the cell wall surface; clumping factor binds nonenzymatically to fibrinogen and platelets, yielding aggregation of the bacteria. The remaining MSCRAMMs, too numerous to mention here (see references), play important roles in establishing S aureus colonization and invasion in major infections such as endocarditis.
Most S aureus strains of clinical importance have polysaccharide capsules, which inhibit phagocytosis by polymorphonuclear leukocytes unless specific antibodies are present. At least 11 serotypes have been identified, with types 5 and 8 responsible for the majority of infections. These capsule types are targets for a conjugate vaccine. Serologic tests have limited usefulness in identifying staphylococci.
Staphylococci can produce disease both through their ability to multiply and spread widely in tissues and through their production of many extracellular substances. Some of these substances are enzymes; others are considered to be toxins, although they may function as enzymes. Many of the toxins are under the genetic control of plasmids; some may be under both chromosomal and extrachromosomal control; and for others, the mechanism of genetic control is not well defined.
Staphylococci produce catalase, which converts hydrogen peroxide into water and oxygen. The catalase test differentiates the staphylococci, which are positive, from the streptococci, which are negative.
S aureus produces an extracellular coagulase, an enzyme-like protein that clots oxalated or citrated plasma. Coagulase binds to prothrombin; together they become enzymatically active and initiate fibrin polymerization. Coagulase may deposit fibrin on the surface of staphylococci, perhaps altering their ingestion by phagocytic cells or their destruction within such cells. Coagulase production is considered synonymous with invasive pathogenic potential.
Clumping factor is cell wall bound and is another example of an MSCRAMM (see earlier) that is responsible for adherence of the organisms to fibrinogen and fibrin. When mixed with plasma, S aureus
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree