(1)
University of South Australia, Adelaide, Australia
Abstract
The conventional method of assessing the value of higher prices of new drugs is the ratio of investment in research and development (R&D) sourced from internal funds to the additional health for the population due to additional future New Molecular Entities (NMEs). There is evidence that the conventionally defined ratio is significantly greater than one. But does this mean that higher prices are necessarily in the population’s interest? A positive correlation between additional future NMEs and future health for the population is a central premise of the prevailing political economy of new drug price. In this chapter I show that a positive correlation is not axiomatic; it is a testable hypothesis and its direction depends on the economic context of the health budget. I present a general expression for the estimate of a return on increased drug prices and public funding for private pharmaceutical R&D that accommodates this context. I distinguish between three types of health budget constraints: (1) a fixed budget that cannot be expanded; (2) a constrained budget that can be expanded incrementally, but only by foregoing the best alternative strategy; and (3) an unconstrained budget that expands to fund every programme that is “cost-effective” in the lay sense of the term. I use this general expression to show that if the health budget is constrained or fixed, even a very high ratio of conventionally defined return to investment does not exclude the possibility that today’s health could have been better had historic prices and R&D been lower.
3.1 The Reimburser’s Problem
A country with a universal health care system and a fixed budget is negotiating a FTA with the US. During the negotiation a US senator visits the country and explains that, even though he is advocating on behalf of the US government, he also has the interest of the citizens of this country at heart. He argues that the evidence is clear; increased life expectancy is driven by new medicines and the availability of new medicines is driven by more pharmaceutical R&D, which is in turn driven by not regulating the FPP. Citizens in all countries would be better off if countries like this one stopped regulating the FPP. The short-term gain of financial savings would be at the cost of the population’s longer-term health; higher prices mean more health.
The Minister for Health and the Minister for International Trade ask the Reimburser her opinion as to whether applying a decision threshold price per effect for new drugs that is lower than the FPP will lead the population’s health to be worse off in the longer run. (The Reimburser makes the final decision regarding the adoption of a new drug at its offer price based on evidence of its additional cost and effect.) The Reimburser is provided with evidence of the gain in average life expectancy at birth in the US; a gain of a full decade over the 60 years from 1950 to 2009. She is also provided with a summary of the peer reviewed evidence of the significance of the contribution of new medicines to this gain (PhRMA 2011). This report concludes that the contribution of new drugs to increased life expectancy in areas such as HIV and cardiovascular disease is in the order of 50–80 %.
The Reimburser is also presented with a study that estimates that the health return on consumer investment in R&D via higher prices (foregone consumer surplus) in the US between 1960 and 2000 is in the order of 28:1 (Santerre and Vernon 2006). A study by Lichtenberg estimates that the social return on pharmaceutical R&D over 1960–2001 was in the order of 160:1 (Lichtenberg 2004). Furthermore, in the concluding chapter of a recent text on the topic of pharmaceutical innovation with contributions from a range of eminent pharma-economists, the editors write that give the evidence suggests that returns are in the order of 10 to 1 that the cost at which developed countries have achieved better health through drugs is much less than the value of this longevity gain.1
Finally, the Reimburser is given a study by Lichtenberg that explores the relationship between drug vintage (the years since patent granted) and Australian improvements in mean age at death and other variables. This study concluded that because their calculations suggest that of the 2 year gain in life expectancy in Australia in the 8 years to 2003, 65 % is attributable to new drugs, that 1.3 (two thirds of 2 years) of this life expectancy would otherwise not have occurred.2
As intuitively appealing as this line of reasoning is, the Reimburser is unsure whether it is sufficient to justify a policy of increased prices of new drugs via a higher threshold. She has these concerns, even though the purported objective of such a policy is the same as the Reimburser’s objective that is, increasing the population’s health. She performs a rough calculation of the estimated additional financial cost to the pharmaceutical budget of the proposed increase in price for new drugs for the next year; a 10 % increase in the pharmaceutical budget. The Reimburser then realises that the additional financial cost to the pharmaceutical budget is permanent; it is not a one-off increase in prices but a policy that would lead to all future pharmaceutical budgets being higher than would otherwise be the case. Furthermore, the policy is expected to lead to more new drugs in the future than would otherwise be the case and they will all be at this higher new price. These additional costs will need to be financed somehow; because the current fiscal climate is one of restraint, other programmes will need to be displaced in order to accommodate these additional costs. While there might be $28 worth of health benefits to certain patients for every additional dollar invested in R&D via higher prices, the ratio of additional population health to additional R&D dollars could be much smaller, or even negative.3 Even if the budget is increased to accommodate these additional costs, other programmes, including the extension of existing programmes, will be foregone. Lichtenberg’s conclusion that the increase in average longevity of a population that is attributable to new drugs would not have occurred in the absence of these drugs is only reasonable under a very restrictive condition: had these new drugs not been purchased from the budget at the higher cost, and older drugs used instead, the savings could not be used to purchase any other services other than drugs that would have also contributed to life expectancy gains.
The Reimburser reviews the US evidence. It seems to her that the basis upon which this return on R&D is estimated makes no reference to these foregone opportunities.4 Can the US pharma-economists and regulators conclude, as they routinely do, that the US population would have been worse off with lower drug prices, if they have not considered the evidence of foregone benefits—the counterfactual? If this evidence of the counterfactual is made available and is found to support the case for higher prices of drugs in the US, can the US trade negotiators claim that all countries will be better off with unregulated (higher) drug prices? The Reimburser wonders if her intuition about the limitations of this evidence has an economic foundation.
The Reimburser asks her Health Economic Adviser:
Does the US estimate of a 28-fold health return on pharmaceutical R&D financed by higher drug prices exclude the possibility that at lower prices and fewer NMEs the US population’s longevity would now be even higher?
3.2 A Closer Look at the Evidence Supporting Pharma’s Lobbying
3.2.1 Why Is Return on Consumers’ Investment in Pharmaceutical R&D Important?
US Pharma sources the majority of its funds for R&D from non-capital market funding: purchasers (via higher prices) and public and private not-for-profit research institutes (Joint Economic Committee 2000; Lichtenberg 2004; Giaccotto et al. 2005). Therefore, US Pharma must lobby (rather than contract with the capital market) to ensure that these funds are at least ongoing, if not increasing. While there is no doubt that increased economic rent for Pharma is an objective of this lobbying for higher price, this is not a politically or socially acceptable justification for non-capital market investment (higher prices). In simple terms, Pharma cannot lobby for higher prices using the following justification: “increase prices because, even though it will increase your organisation’s costs, it will increase our profits”. Instead Pharma must lobby on the basis that there is a return to the funder of this additional R&D, namely, an increase in the population’s future health.
Comanor (1986) observed that the single feature uniting the disparate US pharma-economic literature from the 1959 Kefauver Committee5 to the publication of Comanor’s paper in 1986 was the recognition that the most critical piece of information in the current political economy was an estimate of the return on this investment in pharmaceutical R&D. Comanor also noted three ways in which this return on R&D was defined: the return to the individual firms, the return to the industry and the social return in terms of improved health. It is the last of these three that is of particular interest to the Reimburser and other purchasers and to public health research funding bodies as providers of funds to finance the R&D.
At the time of Comanor’s 1986 publication, no estimate of the social return existed. Comanor reported that one study (Wu and Lindgren 1984) had found social rates of return on three specific new products’ R&D of 65 %, 169 % and 69 %. However, Comanor noted that the study had not deducted “the consumer and producer surpluses obtained from predecessor products” and that “this lead to inflated values”.6
Comanor also noted a practice by US pharma-economists of inferring that the costs of regulation outweighed “any prospective benefits at the regulatory margin”. In simple terms, when some economists identified that there was an unintended negative consequence of increased regulation, they would infer (not prove) that this cost outweighed the benefits of that regulation. For example, increasing the amount of evidence about a new drug that needs to be reviewed by a regulator such as the Food and Drug Administration (FDA) will have the intended consequence of improving safety, but with the unintended consequence of forgone health effects due to the delay in the time for new drugs to reach the market (International Trade Administration 2004 p. 6). Therefore, some pharma-economists might infer that this leads to a net social loss. Comanor identified that the issue is whether the net consequences (identified and unintended) outweigh the benefits that regulation—not simply whether there are unintended consequences.7
If the evidence of the social rate of return, the major justification of ongoing non-capital market investment in R&D, was not available in 1986 then when did it become available?
3.2.2 What Is the Evidence of the Return on Consumers’ Investment?
3.2.2.1 A Review of the Literature
Numerous US pharma-economic studies published between 2000 and 2010 conclude or infer that the result of their study supports the policy of allowing drug companies to price without regulation because society’s return on increased pharmaceutical R&D is high and therefore a population’s future health will be worse if the price of drugs is lowered. However, a detailed review of these studies8 revealed that only two published studies attempted to provide the evidence that is required to inform this policy choice (the return from pharmaceutical R&D estimated as a social return on non-capital market investment). These studies are Santerre and Vernon (2006) and Lichtenberg (2004). So what evidence do these other studies, all of which refer to this return, actually provide?
The remaining studies included in the review were classified into three groups. The first group, which included most of the remaining studies, provided evidence that supports the “policy narrative”, but not the policy choice. The evidence that supports the policy narrative is wide ranging. Some studies indicate that if profit increases, so too does investment in R&D (Vernon 2004). Other studies provide evidence of the high and increasing present value of the costs of bringing a new drug to market (DiMasi et al. 2003). One study demonstrated that even the threat of price control in the US was sufficient to reduce R&D investment (Golec et al. 2005). The second group of studies provided evidence of rate of return estimates for the purchase of new drugs (not drug R&D). For example, retrospective analysis of historic data showed that increased expenditure on new drugs led to a health benefit with a monetary value greater than the additional cost of these drugs (Cremieux et al. 2007).9 The third group comprised three studies which could, under very restrictive conditions, be interpreted as providing evidence of the social rate of return; however, these conditions were very unlikely to occur.
3.2.2.2 The Two Published Estimates of the Social Return to Consumers’ Investment in High Prices
The two studies published between 2000 and 2010 that estimated a return on the ongoing non-capital market investment in pharmaceutical R&D found a very high return on historic investments of consumer surplus in pharmaceutical R&D via higher prices; in the order of 28-fold from Santerre and Vernon’s study and 160-fold from Lichtenberg’s study. These estimates of return are a powerful piece of evidence supporting continued and increased investment in pharmaceutical R&D via non-capital market sources. With returns this high, why would a rational institution respond to Pharma’s lobbying in any way other than continuing and possibly increasing this non-capital market funding? A closer review of these ratios reveals that they do not accommodate the forgone opportunities of alternative investments in health R&D (e.g, clinical trials of new service delivery models) or other ways of improving health outcomes (e.g. expanding workforce). Hence these ratios do not accommodate the full cost to society of higher prices.
The expression underlying Santerre and Vernon’s estimate is generalised to the following form:
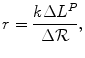
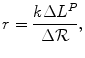
(3.1)
where k is the maxWTP for an additional year of life (and assumed to be constant regardless of the investment in R&D); ΔL P is the additional life-years possible from new drugs and Δℛ is the additional investment in R&D, which leads to the development of additional drugs. Hence, a 28-fold return on consumers’ welfare means that for every dollar of revenue from higher prices (foregone consumer welfare), the additional life-years from the additional new drugs had a monetary value of $28. This general expression is the measure of rate of return that is consistent with the prevailing political economy; the cost of increased health budget savings (reduction in ℛ) is decreased life-years in the future (reduction in L P ).
The policy narrative states (with supporting evidence) that: (1) increased R&D will lead to more drugs; and (2) drugs have an average impact on life expectancy that is greater than or equal to zero. Therefore, if prices and R&D increase, so too must the population’s future health. If the policy narrative is accepted, then it is reasonable that this expression of rate of return excludes the possibility that reduced R&D will improve the population’s health. However the central premise of the prevailing political economy (that the trade-off between price and future health exists) is not tested by the empirical research. Specifically, Eq. (3.1) does not accommodate the possibility that: (1) the net health effect of the drug for target patients could be positive and higher prices and hence more R&D could lead to more new drugs; but (2) the net effect of more R&D on the population’s health could be negative. This finding is consistent with the observation by Comanor (1986) that the possibility that improved competition (lower prices) today and improved future health could both occur is not considered in the research agenda.
A more general expression of this return on non-capital market investment in pharmaceutical R&D would accommodate the possibility that policy can both improve competition (lower prices) and improve the population’s health both today and in the future. However, before such an expression can be developed, the critical implicit assumption in the US pharma-economic literature needs to be explicated. This assumption concerns the nature (and/or the existence) of the budget constraint.
3.3 Fixed, Constrained and Unconstrained Budgets
In the simplest sense, budget constraints can be fixed, absent or something “in between”. To explore the question of “in between” we need a formal distinction between the different types of budget constraints. Such a distinction is proposed by Claxton et al. (2000). In the context of a discussion about the shadow price of the budget constraint,10 Claxton et al. raised the idea of two types of budgets for health. The magnitude of the first is defined by some decision maker external to the health sector, for example a Treasury Department official who takes into account the constraints in total government spending. This budget is set at a fixed amount and cannot be expanded. The second type of budget has a size that is defined by a decision about the maxWTP for a health effect. Any programme that meets the defined threshold can be financed, and all programmes that meet this criterion are financed. In this case, the budget expands to accommodate any programme that meets this criterion. Claxton et al. describe the latter budget as endogenous and the former as exogenous. But is this distinction between two types of budgets sufficient? The following section defines four distinct types of budgets.
3.3.1 Four Types of Budgets
In an adaption of Claxton et al., the following formal definitions of budget constraints are proposed and used throughout this book.
1.
A fixed budget cannot be expanded and any additional purchases can be funded only if an existing activity is displaced.
2.
A constrained budget can be expanded by a trigger such as the decision to finance a new drug but there is a foregone benefit to the expansion to finance a drug; other health and non-health programmes or investments in R&D for unpatented programmes could instead have been expanded or implemented.
3.
An unconstrained budget is one that is expanded to accommodate any purchase that has an ICER at or below the maxWTP, where this maxWTP is defined by the social decision maker as in the endogenous budget from Claxton et al. (2000). The corollary of this definition of a budget is that there is no foregone benefit, within or external to the health sector, to any purchase with an ICER at or below that threshold. The price of a new drug is relevant to the new drug adoption decision in that the price must not be above the maxWTP.
4.
No budget means that there is no constraint on health expenditure. The price of a new drug is not relevant to the new drug adoption decision in this context.
3.3.2 Why Is It Useful to Have Four Classifications of Budgets?
The important point about these distinctions is their implication for interpreting the benefit of new drugs. The US pharma-economic literature generally implicitly assumes that the health budget is unconstrained. In this context it is reasonable to conclude that if new drugs can be shown to have contributed to improved health of target patients in the past, then having fewer new drugs in the future will reduce the population’s future health.
An example of the application of this assumption in the peer reviewed literature is Lichtenberg and Duflos (2008). The authors state that an empirical finding that 65 % of a 2-year increase in life expectancy over the period 1995–2003 can be attributed to new drugs means that if these new drugs had not been available, the increase in the life expectancy of Australians would have been 35 % of this amount, namely 0.7 years, over this period. This implicitly assumes that there is no other opportunity to improve the patient’s health, other than the use of new drugs. However, the only condition under which this claim can be made is if we assume an unconstrained budget; all other opportunities to improve health are already funded because they meet the decision threshold. Under a constrained or fixed budget, the resources allocated to the additional cost of new drugs could have otherwise been allocated to other programmes. The counterfactual to additional expenditure on new drugs would have been a world in which other services were purchased, resulting in health benefits that were either better than, less than or no different from those from the availability of more new drugs. In the context of the unconstrained budget, this counterfactual is irrelevant; if the counterfactual programme had an ICER less than or equal to the maxWTP, then it would have already been funded, and its funding would not be dependent upon the expenditure on new drugs.
Using these distinctions between types of budgets we can now prove that Santerre and Vernon’s result of a 28-fold return on consumer investments via higher prices only excludes the possibility that the US could have done better with lower prices if there is an unconstrained budget. But first we start with a general expression for rate of return that accommodates all of these budgets.
3.4 Accommodating the Budget Constraint in the Return on R&D
There is more than one possible expression for a return on R&D funded by non-capital market funds, and a number will include the benefits of foregone activity, which, if it is the best alternative activity, is the opportunity cost. One general expression that accommodates the possibility that more drugs could either increase, decrease or not impact on a population’s future health is presented in this section. I then show that the conventional measure of the rate of return, r [Eq. (3.1)] is a special case of the general expression, where the budget is unconstrained. Finally, I derive the conditions under which the population’s future health increases if both current prices and the number of future NMEs decrease.
3.4.1 A General Expression for the Rate of Return on Consumer Investment in Pharmaceutical R&D
One general expression for return on non-capital market sourced investment in pharmaceutical R&D is:
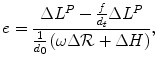
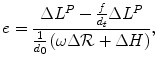
(3.2)
where:
ΔL P is the additional health effects (L) possible from the additional new drugs (P);
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


