Local and unit-specific antimicrobial guidelines need to be constructed after considering the above mentioned factors. Use of such guidelines increases the chance of achieving adequate empiric coverage. Such guidelines have to be updated regularly after considering changes in the local microbiologic flora and resistance patterns.
Combination Antibiotic Therapy
Combination therapy is sometimes required as initial empirical therapy in order to cover multiple possible pathogens commonly associated with the specific infection. Frequently combination therapy with different modes of action are used (eg., beta lactam/aminoglycoside, beta lactam/fluroquinolone, beta lactam/macrolide-clindamycin combinations). If one of the antibiotics is already sensitive, the incremental role of the second antibiotic that is also sensitive is uncertain. Combination therapy has been shown to be beneficial in animal models, and in certain diseases such as endocarditis and cryptococcal meningitis. However meta-analyses have failed to demonstrate evidence of benefit for combination therapy.
De-escalation of Antibiotic Therapy
As a consequence of high mortality associated with inappropriate initial empiric therapy, these antibiotics have to cover a broad spectrum of pathogens. Continued use of such broad spectrum antibiotics predisposes to recurrence of infections with multi resistant pathogens in the same patient, increases the risk of clostridium difficile infection and contributes to general increase in resistance problems in the unit as a whole. So the empiric broad spectrum therapy should be aggressively de-escalated within 48–72 h if a plausible pathogen is identified. This can be achieved by changing the antimicrobial to one that has narrower spectrum of cover or by converting combination therapy to monotherapy. In patients who are culture negative, de-escalation is still possible after the patient stabilises clinically (i.e., after shock resolution). Such an approach will maximise the chances of achieving appropriate empirical antimicrobial therapy while minimizing selection pressure for resistant organisms.
Duration of Antibiotic Therapy
Duration of antimicrobial therapy has traditionally been based on expert opinion. With the increased awareness of the harmful effects of prolonged courses of antimicrobials, current trend is to give shorter courses of treatment. For example, for ventilator associated pneumonia, an 8 day course is as effective as 15 day course. Patients on shorter course of antibiotics develop fewer recurrent infections with multi-resistant pathogens. However, in some conditions such as endocarditis, osteomyelitis and intra-abdominal abscesses, longer regimes of 4–6 weeks are needed. In many such situations, the treatment duration has to be individualised after carefully considering the clinical and radiological response.
Dose Optimization in Critically Ill Patients
It is important to understand the many factors that affect the amount of antibiotics arriving at the target tissue in the acutely ill patients (see Chap. 6). Adjustment of doses and choices of antibiotics with these factors taken into consideration will more often result in a better outcome.
Antibiotic Classification in Sepsis
Antibiotics can be classified by their affinity for water to hydrophilic and lipophilic antibiotics (Table 25.2). Knowing this will allow the provider to understand how far the antibiotic will reach in the various types of tissues. They can also be classified by their pharmacodynamics into concentration-dependent antibiotics, time-dependent antibiotics and concentration-dependent antibiotics with time dependence (Fig. 25.1). These will give rise to apparent volume of distributions (Vd) which are different for various antibiotics.
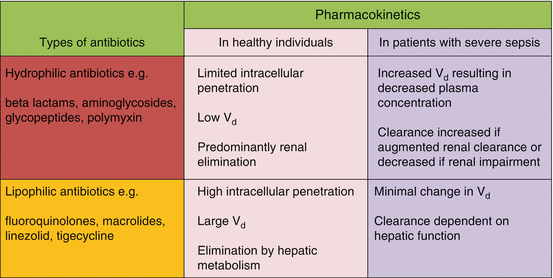
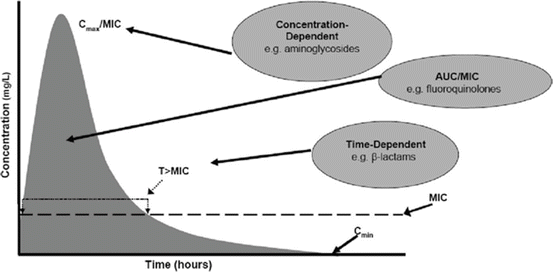
Table 25.2
Types of antibiotics based on affinity for water


Fig. 25.1
Types of antibiotics based on pharmacokinetics of the drugs (With permission from Wolters Kluwer Health Critical Care Medicine 2008; 36(8):2433–40)
Hydrophilic antibiotics are well distributed in both the intravascular and interstitial space (the extracellular space) and are not available in the intracellular space in meaningful concentrations. So their volume of distribution (Vd) is equivalent to the extracellular water.
Lipophilic antibiotics are available both in the intracellular space and in adipose tissues since they can cross lipid membranes. So their Vd is dependent on the amount of adipose tissue which is in turn dependent on the total body weight of the individual.
For concentration-dependent antibiotics the optimal activity correlates with ratio of peak serum concentration (Cmax) with minimum inhibitory concentration (MIC) of the bacteria (Cmax/MIC) (Fig. 25.1). For time-dependent antibiotics, the optimal activity correlates with the proportion of time the dosing interval the unbound drug (fT) is maintained above the MIC of the bacteria (fT > MIC). For concentration-dependent antibiotics with time dependence, it is the ratio between unbound fraction area under the curve of the drug (fAUC0–24) and the MIC of the bacteria (fAUC0–24/MIC).
Pharmacokinetic and Pharmacodynamic Considerations
Dosing
The Vd in critically ill patients with sepsis is increased due to the capillary leak syndrome which results in the shifting of fluids to the interstitial space. In critically ill patients, the Vd is also increased secondary to mechanical ventilation, fluid resuscitation, post-surgical drains, hypoalbuminemia (which causes plasma leakage) and extracorporeal circuits such as cardiopulmonary bypass. The increase in Vd has a dilutional effect on hydrophilic drugs resulting in lowering of their plasma concentration. This dilution effect has little effect on lipophilic antibiotics. However obesity increases the Vd of lipophilic drugs.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


