Fig. 21.1
Pretreatment pure-tone audiogram of a patient presenting with right profound sudden sensorineural hearing loss
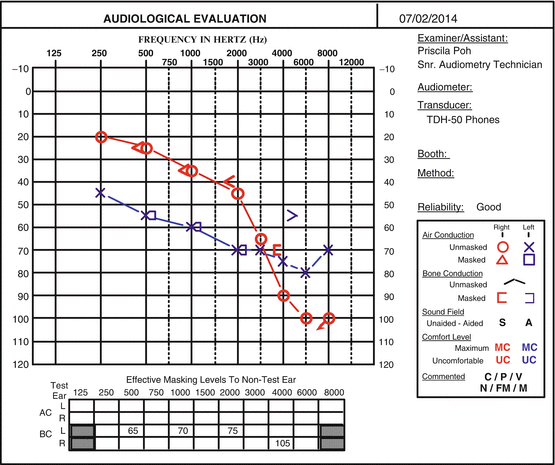
Fig. 21.2
Posttreatment audiogram of the same patient treated with a course of intratympanic methylprednisolone showing good recovery in the low and mid (but not the higher) speech frequencies
21.1.5 Combined Steroid and Antioxidant Treatment in ISSNHL
Based on the potential therapeutic effects of steroids and antioxidants, the addition of antioxidants to steroid treatment may yield better outcomes in ISSNHL. Oxidative stress is known to be a key determinant in dysfunction of the vascular endothelium (Capaccio et al. 2012). Beneficial effects of steroids are thought to be due to their anti-inflammatory properties. Steroid-responsive hearing disorders may also be attributed to vascular disruption in the stria vascularis, which may lead to a breakdown of the blood-labyrinth barrier and endolymph ion homeostasis (Trune and Canlon 2012). Hence, antioxidants and steroids may well have complementary or even synergistic effects in improving vascular function. On the other hand, although glucocorticoids confer antiapoptotic properties in the hair cells, there is only weak expression of glucocorticoid receptors in OHCs (Trune and Canlon 2012; Amsterdam et al. 2002). Therefore, the prevention of apoptosis of OHCs might be better effected by antioxidants than by steroids.
21.1.6 Conclusion
The pathogenesis of ISSNHL and its treatment are controversial, although there is now good evidence to support the use of steroids delivered by the intratympanic route. In recent years, there has been a growing body of evidence to suggest that free oxygen radicals play an important role in ISSNHL and that antioxidant treatment might be of benefit. Further well-designed randomized blinded studies are warranted to find out if the early addition of antioxidants to standard steroid therapy can enhance recovery of ISSNHL, particularly in the less treatment-responsive high-frequency hearing losses.
21.2 Meniere’s Disease
Meniere’s disease (MD) is an inner ear disorder characterized by spontaneous episodes of vertigo lasting at least 20 min, accompanied by fluctuating low-frequency sensorineural hearing loss, aural fullness, and tinnitus. Endolymphatic hydrops (ELH) was first noted in the temporal bones of patients suffering from Meniere’s syndrome in 1938. However, recent work has shown that ELH is a histologic marker of MD rather than the direct pathophysiologic cause (Merchant et al. 2005). Dysregulation of endolymph, infectious, autoimmune, vascular, genetic, dietary, endocrine, and many other etiologic factors have been hypothesized to result in ELH (Kiang 1989). The membrane potential of sensory cells found within the inner ear is regulated by K+ selective channels and the cycling of K+ from endolymph to perilymph and back helps to generate the electrochemical gradient needed for sensory transduction (Wangemann 2002). Fibrocytes within the cochlea are involved in this K+ cycling. In surgically blocked endolymphatic sacs of guinea pig, type 1 fibrocytes showed osmotic stress with an increase in Na–K–2Cl cotransporter (NKCCl) and decrease in taurine and C-Jun N-terminal kinase (JNK) (Semaan et al. 2005; Shinomori et al. 2001).
While a major pathophysiologic theory of MD etiology invokes rupture of the membranous labyrinth with resultant mixing of endolymph and perilymph and end organ damage, ultrastructural evidence instead points to neuronal injury, lending credence to the theory of neurotoxicity (Semaan et al. 2005). There is increasing evidence that oxidative stress plays a critical role in pathogenesis of MD.
Nitric oxide (NO) is generated by nitric oxide synthase (NOS). Type 1 (brain/neuronal) and Type 3 (endothelial) NOS are activated by calcium-ion influx, are constitutive, and produce small amounts of NO. In contrast, Type 2 (inducible) NOS is calcium ion independent, and can produce 100–1,000-fold more NO than the other types. Nitric oxide has been noted in afferent nerve endings, inner and OHCs, the organ of Corti, stria vascularis, spiral ligament, and the spiral vessel to the basal membrane in the guinea pig model (Shi et al. 2001). Injection of keyhole limpet hemocyanin into the endolymphatic sac of guinea pigs simulated ELH and revealed that inducible Type 2 NOS is found within the hydropic vestibule, especially in the sensory epithelium and vestibular ganglion cells (Watanabe et al. 2001). NO release results in increased release of excitatory amino acids such as glutamate; these peaks of NO release are present during both early and late phase of tissue damage (Takumida and Anniko 2002). Guinea pig models have implicated glutamate in ELH, with upregulation of NOS and caspase (Anne et al. 2007). In general, NO generation results in glutamatergic activation of N-methyl-d-aspartate (NMDA) receptors. Chronic glutamate overstimulation of the NMDA receptor leads to calcium influx, with the ensuing cellular stress leading to activation of the caspase cascade and NO production (Orrenius et al. 2003). Loss of type 1 spiral ganglion neurons through glutamate excitotoxicity has been implicated as the main pathologic finding with hearing loss found in ELH (Megerian 2005).
Stress-induced ROS, free radicals, and caspase can be measured in the spiral ganglion cells, stria vascularis, fibrocytes, organ of Corti, and blood vessels of the spiral ligament in surgically hydropic guinea pigs (Labbe et al. 2005). The resultant damage to mitochondrial membrane potential leads to cytochrome c release and cleavage of procaspase-9 and further downstream activation of caspase-3 (Van De Water et al. 2004, see also Chap. 19, by Van De Water). While caspase-9, caspase-3, and to some extent caspase-8, are activated through this intrinsic mitochondrial cell death pathway and participate in the normal development and maturation of the membranous labyrinth and cochleovestibular ganglion, post development, they are also implicated in the cause of cell death and propagation of ELH. High volumes of ROS lead to programmed cell death (Bras et al. 2005).
Cytoprotection from ROS are represented by vitagenes, including heat shock proteins (Hsps) heme-oxygenase 1, and Hsp-70 (Calabrese et al. 2010). There is ongoing research to explore intrinsic capabilities of GSH to control redox homeostasis along with exogenous medications to control oxidative stress within the inner ear (Abi-Hachem et al. 2010). Riluzole, a glutamate release inhibitor, originally developed to treat amyotrophic lateral sclerosis (Hugon 1996), has been shown to at least reduce hearing loss related to acoustic trauma (Wang et al. 2002). Dimethylsulfoxide (DMSO), a potential carrier for riluzole, independently and reversibly inhibits NMDA, suppresses calcium influx produced by glutamate and scavenges ROS, which can prevent apoptosis (Lu and Mattson 2001). Melki et al. (2010) used the Phex Hyp–Duk mouse, which spontaneously develops ELH and postnatal hearing loss to study possible protective effects of riluzole and DMSO. Mice were treated, beginning at postnatal day 6, with either riluzole and DMSO (required carrier), DMSO alone, or no-injection control. The authors demonstrated a statistically significant protective effect against hearing loss with DMSO, independent of riluzole, at least in the dosage used. DMSO has antioxidant properties and its potential sites of action are illustrated in Fig. 21.3 (Melki et al. 2010).
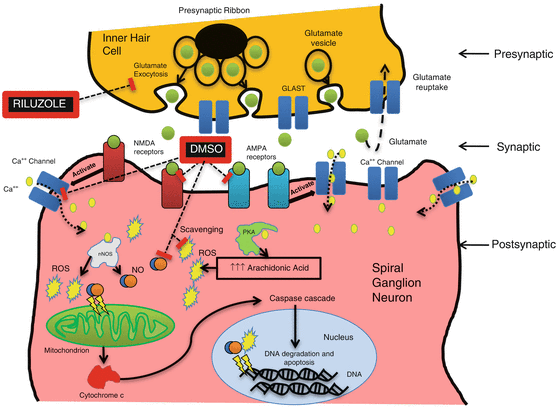

Fig. 21.3
Glutamate excitotoxicity pathway and potential site of action of riluzole and DMSO. Riluzole acts by blocking glutamate release and DMSO by antagonizing glutamate’s action by inhibiting NMDA and AMPA receptors and diminishing the calcium influx into the cell by (hypothetical) modification of the characteristics of the calcium channels. DMSO also acts as a scavenger of ROS. PKA protein kinase A; nNOS neuronal nitric oxide synthase; NO nitric oxide (reproduced from Melki et al. 2010 with permission)
21.2.1 Conclusion
Evidence shows that while glutamate is an important excitatory amino acid within the inner ear, glutamate excitotoxicity results in increased oxidative stress. This initiates downstream apoptotic processes within type 1 spiral ganglion cells which may be responsible for eventual irreversible hearing loss in Meniere’s disease. While more research is needed, there is hope that antioxidants may prevent or reduce permanent hearing loss associated with this disorder. This has great potential clinically as therapeutic hearing preservation has so far been an elusive goal in the management of Meniere’s disease. Given the current level of evidence of possible efficacy and the safety profile of many of the antioxidants available, clinical trials to assess their utility would seem to be warranted at this time.
References
Abi-Hachem RN, Zine A, Van De Water TR (2010) The injured cochlea as a target for inflammatory processes, initiation of cell death pathways, and application of related cytoprotective strategies. Recent Pat CNS Drug Discov 5(2):147–163PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


