Fig. 3.1
Glucose is phosphorylated upon cellular entry and isomerized to fructose-6-phosphate (F-6-P) prior to utilization through glycolysis and pentose phosphate pathway (PPP). Up to 5 % of F-6-P however is converted to glucosamine-6-phosphate (GlcN-6-P) by the rate-limiting enzyme GFPT and subsequently acetylated by glucosamine-6-phosphate acetyl transferase (Emeg32) and converted into UDP-N-GlcNAc via the UDP-GlcNAc pyrophosphorylase (uap1) for use as a donor substrate in multiple biosynthetic reactions including the O-linked modification of a diverse group of nucleoplasmic proteins by N-acetylglucosamine (GlcNAc). Addition of GlcNAc to ser/thr residues of target proteins is catalysed by O-GlcNAc transferase (OGT) and its removal by an O-GlcNAc-selective β-N-acetylglucosaminidase (O-GlcNAcase or OGA). These reactions can be manipulated using specific inhibitors, BADGP and PUGNAc to evaluate the role of O-linked GlcNAcylation systematically, whilst the rate-limiting step in UDP-GlcNAc formation catalysed by GFPT, can be manipulated experimentally using the glucosamine analogue azaserine
Unlike other nutrient-response systems that respond to specific stimuli, for example AMPK, which responds to the AMP/ATP ratio, and mTOR which responds to amino acid concentrations as well as to the insulin/PI3K system, the HBP integrates several nutrient sources and nutrient sensing pathways, making it acutely sensitive to all nutrient flux (Hanover et al. 2010). Indeed, the HBP integrates the metabolism of carbohydrates (glucose), amino acids (glutamine), fat (acetyl CoA) and nucleotides (uridine-diphosphate) with energy charge (ATP) in the synthesis of UDP-GlcNAc, making this activated sugar-nucleotide potentially the most general sensor of cellular nutritional status (Hanover et al. 2010). UDP-GlcNAc then acts as the donor substrate for a number of biosynthetic reactions including GPI lipid anchor biosynthesis, sugar nucleotide, glycoside and ganglioside biosynthesis and N-linked glycosylation for the production of glucosaminoglycans, glycolipids and membrane and secretory glycoproteins. Additionally UDP-GlcNAc acts as a precursor to modify regulatory proteins post-translationally in the nucleus and cytoplasm through O-linked glycosylation with N-acetyl glucosamine (O-GlcNAcylation) (Fig. 3.1).
Not surprisingly, the HBP is highly active during oocyte maturation where it is utilised for the biosynthesis of complex glycan structures such as hyaluronic acid in the extra cellular matrix of cumulus-oocyte complexes (COCs). Indeed, it is estimated that 25 % of glucose consumed by gonadotropin stimulated bovine COCs is incorporated into the extracellular matrix via the hexosamine biosynthetic pathway to facilitate cumulus expansion (Sutton-McDowall et al. 2004).
3.5 The Response Path: N-Linked Vs. O-Linked Glycosylation?
Early studies in embryos using tunicamycin, showed that this specific inhibitor of N-linked glycosylation suppressed glucose incorporation into glycoproteins and inhibits compaction (Surani 1979; Wales and Hunter 1990). However, it did not affect other metabolic pathways including glycolysis (Wales and Hunter 1990) suggesting that metabolic differentiation proceeds normally in these embryos in direct contrast to the effects of glucose deprivation.
Interestingly [14C]-amino acid incorporation into the embryo glycoprotein fraction was found to be insensitive to tunicamycin treatment (Wales and Hunter 1990). Moreover an earlier study using 3H-glucosamine showed that 40 % of the incorporated glycoprotein label in blastocysts is also tunicamycin insensitive (Surani 1979). Taken together these data suggest that N-linked glycosylation is unlikely to be the responsive mechanism at the heart of glucose activated blastocyst formation. The most likely downstream effector pathway in activating an adaptive response and metabolic differentiation is therefore most likely to be O-linked modification of cellular proteins by N-acetylglucosamine. Studies in bovine oocytes implicating O-linked glycosylation in oocyte developmental competence (Sutton-McDowall et al. 2004) were also consistent with this pathway playing a critical role in glucose signaling in early development and metabolic differentiation associated with blastocyst formation.
In contrast to the Golgi associated N-glycosylation of membrane associated or secreted glycoproteins and other forms of glycosylation, O-linked glycosylation (or O-GlcNAcylation) is a nuclear and cytosolic modification of an increasingly diverse range of nuclear and cytoplasmic proteins (Holt and Hart 1986). It is a short (single residue) regulatory modification exhibiting properties similar to phosphorylation in contrast to other typical forms of glycosylation (Kreppel et al. 1997) which typically exhibit multiple, often branched glycosidic residues and are associated with cell-cell interactions. Target proteins are modified by a dynamic process involving cyclic addition and removal of a single moiety of N-acetylglucosamine (GlcNAc) to the hydroxyl groups of serine or threonine residues. This latter, terminal signal-transducing arm of the hexosamine biosynthetic pathway is now known as the hexosamine signaling pathway (HSP). In terms of high-energy compounds, the intracellular concentration of the activated donor, UDP-GlcNAc, is second only to ATP, making O-GlcNAcylation one of the most common cellular post-translational modifications (Hart et al. 2007).
3.6 Hexosamine Signalling: A Nutrient Response Pathway
3.6.1 The Enzymes
The enzyme involved in the terminal transfer of the GlcNAc moiety to target proteins is the O-linked N-acetylglucosaminyltransferase (OGT) while the removal of GlcNAc is catalyzed by the β-selective N-acetylglucosaminidase (O-GlcNAcase or OGA- annotated as meningioma-expressed antigen mgea5 and characterized as a hyaluronidase (Heckel et al. 1998)) (Wells and Hart 2003; Zachara et al. 2004). These form a single cooperatively regulated enzyme complex whose activity is exquisitely sensitive to UDP-GlcNAc and hence nutrient supply (Kreppel and Hart 1999). Both enzymes are products of single, highly conserved genes with differentially spliced variants in mammals.
OGT maps to Xq13 on the mammalian X-chromosome and encodes three variants which differ in the length of their amino terminal tetratricopeptide (TRP) repeats (which vary from 3–12 repeats) and in their subcellular distribution: short OGT, mitochondrial OGT (mOGT) and nuclear/cytoplasmic OGT (ncOGT) (Lubas et al. 1997; Kreppel et al. 1997; Kreppel and Hart 1999; Lubas and Hanover 2000). The TRP domains produce a superhelical structure containing an asparagine ladder that is critical for protein recognition (Jinek et al. 2004). Alternate splicing of these differentially targeted variants therefore produces isoforms with varying TRPs thus allowing each isoform to modify a select subset of substrates, conferring on this single gene enzyme the ability to modify many targets (Lubas et al. 1997; Kreppel et al. 1997; Lazarus et al. 2006).
OGA exists as two isoforms: the full-length isoform incorporates a histone acetyltransferase (HAT) domain in its C-terminus and a short isoform lacking the C-terminal HAT domain. The shorter isoform is associated with lipid droplets and thought to have a role in lipid droplet assembly and mobilization, since it promotes proteasomal degradation of surface lipid droplet proteins (Keembiyehetty et al. 2011). The longer isoform, on the other hand, is present throughout the nucleus and cytoplasm. Moreover, this bifunctional nuclear/cytoplasmic OGA associates with OGT through specific domains into a single O-GlcNAczyme complex (Whisenhunt et al. 2006). Basal OGT and OGA activities are modulated by their own intrinsic O-GlcNAcylation status (Kreppel et al. 1997; Gloster and Vocadlo 2010) supporting the idea of cooperative activity between the two enzymes. Ultimately this co-operativity results in a finely balanced equilibrium whereby even small deviations in O-linked glycosylation impact on cellular signalling activities and therefore cellular homeostasis in response to a nutrient signal.
OGT and OGA appear to regulate attachment and removal of O-GlcNAc in much the same way that kinases and phosphatases regulate phosphorylation. OGT was originally believed specifically to target serine/threonine residues on a number of nucleoplasmic proteins to alter their activity and stability in response to nutrient availability (Wells et al. 2001). Indeed, for a subset of proteins, OGT competes dynamically with protein kinases leading to the idea that such modification is functionally reciprocal to phosphorylation at the same sites (Kelly et al. 1993; Comer and Hart 2001; Chou et al. 1995). Whereas protein phosphorylation is achieved by the coordinate regulation of approximately 500 protein kinases and ~ 100 phosphatases (Forrest et al. 2003; Manning et al. 2002) the regulation of O-GlcNAcylation is achieved by the concerted action of the two highly conserved enzymes, OGT and OGA, highlighting their potential significance as regulators of kinase dependent cellular pathways.
3.6.2 The Targets
Whilst this reciprocity certainly exists for a subset of proteins and is well characterized for some, such as the RNA polymerase II C-terminal domain (Comer and Hart 2001) and Sp1 (Kudlow 2006), for other proteins such as p53, O-GlcNAcylation can sterically hinder nearby phosphorylation sites and impact on their activity (Yang et al. 2006). The number of proteins whose activity and stability is modified by O-linked glycosylation is extensive and increasing and includes transcription factors, nuclear pore proteins, cytoskeletal components, metabolic enzymes as well as phosphatases and kinases that are known regulators of early developmental processes. There is in fact extensive cross-talk between OGT and the canonical nutrient sensing kinase cascades such as Insulin-Akt, MAPK, mTOR and AMPK (Hanover et al. 2010). Whilst O-linked glycosylation often results in decreased protein activity this is not a generalised phenomenon since in some proteins such as the global transcription factor Sp1 GlcNAc modification can increase protein activity (Goldberg et al. 2006; Wells and Hart 2003). Moreover, studies also show that O-linked glycosylation can both prevent and increase target susceptibility to proteasomal degradation (Han and Kudlow 1997). The 26S proteasome is a proteolytic organelle present in both the cytoplasm and nucleus responsible for degradation of proteins that are marked for turnover by polyubiquitination, thus controlling protein half-life, maintenance of metabolic proteins and elimination of damaged proteins. It is present throughout preimplantation development and its function is essential for early embryonic development (McCue et al. 2008). In the case of Sp1, under conditions of inadequate nutrition, O-GlcNAcylation of this transcription factor is reduced and this correlates with increased degradation that can be inhibited by proteasomal inhibition (Han and Kudlow 1997). Conversely hyperglycosylated Sp1 is protected from proteasomal degradation leading to the suggestion that O-GlcNAcylation of Sp1 may play a role as a nutritional checkpoint by generally reducing transcription in the absence of adequate nutrition (Han and Kudlow 1997). Consistent with this hypothesis, Sp1 promoter activation is reduced in an O-GlcNAc dependent manner as a result of an interaction between OGT and the transcriptional repressor mSin3A (Yang et al. 2002). Inhibition of the proteasome itself by O-linked glycosylation also couples protein turnover to metabolic state allowing cells to control the availability of amino acids and regulatory proteins (Zhang et al. 2003).
O-GlcNAcylation and/or the enzyme OGT, can also interact with other post-translational modifications such as ubiquitination (Guinez et al. 2008), which targets cellular proteins to the proteasome, and nitrosylation (Ryu and Do 2011) although the mechanisms by which these interactions occur are not clearly defined. Moreover, the OGT/OGA complex associates with histone deacetylases (HDACs) in nuclear transcriptional co-repression complexes to regulate transcription. Disruption of the enzyme complex regulating O-GlcNAcylation in these complexes interferes with transcriptional repression (Whisenhunt et al. 2006), thus implicating the enzyme complex in still broader roles. More recently OGT and O-GlcNAcylation have also become linked to the multi-faceted “histone code” with recent findings suggesting that all four core histones are modified by O-GlcNAc (Sakabe et al. 2010). The sites of O-GlcNAc-histone modification hint at a role in chromatin remodeling and add to a mounting body of evidence linking O-GlcNAc cycling to higher-order chromatin organization and epigenetic memory (Hanover et al. 2012). The identity and function of these targets and partners would suggest that HSP plays a critical role in controlling all aspects of cellular function ranging from protein activity and stability, fuel metabolism, cytoskeletal organization, cell growth, gene expression and differentiation as well as longer-term transcriptional regulation of developmental processes in response to nutrient availability.
3.7 O-GlcNAcylation in Development
Unlike many other glycosyltransferases, OGT is a soluble protein predominantly found in the nucleus but associated with nuclear pores, mitochondria and cytoplasm of all tissues studied to date (Kreppel et al. 1997; Haltiwanger et al. 1992; Hanover et al. 2003). Consistent with this distribution, mouse embryos pulse-labelled with 14C-glucosamine display 14C-label grains in the nucleolus, regions of nucleolar-associated chromatin and the nuclear envelope (Acey et al. 1977). This is consistent with observations of predominantly nuclear but also cytoplasmic associated O-GlcNAc in early mouse embryos using antisera that recognize the β-O-GlcNAc linkage (Pantaleon et al. 2010). Interestingly GLUT1 is also nuclear at this stage in development (Pantaleon et al. 2001a) and may provide a potential conduit for hexosamines to this location, although direct evidence for this is currently not available.
Deletion of key enzymes involved in the HSP is found to be lethal in mammals (Boehmelt et al. 2000; O’Donnell et al. 2004; Shafi et al. 2000; Yang et al. 2012). OGT is an X-linked gene, and since most mouse embryonic stem cell lines are XY, early attempts to generate knockout mice were unsuccessful as they resulted in stem cell lethality (Shafi et al. 2000). This necessitated the use of Cre-recombinase technology to generate conditional, tissue-specific OGT deletion thus highlighting the fundamental importance of O-GlcNAcylation in embryo viability . Tissue-specific knockouts of OGT lead to profound changes in all cells types examined including thymocytes, and fibroblasts, resulting in T-cell apoptosis, and fibroblast growth arrest, respectively. Moreover, the neuronal specific knockout results in smaller pups with abberant locomotor activity. These animals did not nurse well and died about 10 days post-natally (O’Donnell et al. 2004). Targeted OGT deletion in the ooyte is also embryonic lethal resulting in peri-implantational (E5) abortion (O’Donnell et al. 2004) clearly indicating that preimplantation development is severely compromised. Taken together these outcomes suggest essential, pleiotropic roles for this enzyme during development.
3.8 The HSP: Sensor of an Adverse Environment?
Given the ability of glucose signaling through this pathway to impart adaptive capacity to the developing embryo as discussed earlier and the functional diversity of the potential targets, the critical role played by this signaling pathway and O-GlcNAc modification mostly likely relates to maintenance of cellular homeostasis in response to stress as part of a pro-survival signaling program. As such it may be viewed as a potential sensor of an adverse environment.
Indeed, blocking hexosamine biosynthesis and hense the sensor component of this pathway eliminates stress responsiveness leading to a decrease in cellular survival in numerous systems (Zachara and Hart 2006). This is consistent with observations in the early embryo discussed earlier, where inhibition of GFPT ablates the ability of glucose to activate embryonic adaptive responses (Pantaleon et al. 2008). Blocking or reducing O-GlcNAcylation and thus cellular capacity to respond to stress also renders cells more vulnerable to stress and decreases cellular survival, whilst increasing levels of O-GlcNAcylation appear to have a protective effect by promoting cell survival (Zachara et al. 2004).
Indeed, in somatic cells, cellular stress is intimately linked with changes in O-GlcNAcylation which, in response to multiple forms of cellular stress , appears to increase in a dose dependent manner in cultured cells (Zachara et al. 2004). Significantly, increasing levels of O-GlcNAc prior to (Zachara et al. 2004) or immediately following, cellular injury promotes survival whilst suppressing them sensitizes cells to death suggesting that this a key regulator of the cellular stress response (Zachara and Hart 2004).
Stress induced O-GlcNAcylation promotes cell and tissue survival by regulating a multitude of biological processes which include but are not limited to, the phosphoisitide/Akt pathway, central to survival signalling, heat shock protein expression, calcium homeostasis and reactive oxygen species (ROS) generation as well as mitochondrial dynamics (Zachara and Hart 2006). These are all aimed at maintaining ATP levels, mitochondrial membrane potential and stabilizing redox state: all critical elements of early embryo viability (Lane and Gardner 2005). Ubiquitination and proteasomal degradation of p53 , the most extensively studied tumor suppressor, is blocked by O-GlcNAcylation (Yang et al. 2006). This observation is relevant to survival signaling in the preimplantation embryo, which involves stimulation of pro-survival factor expression (mitochondrial Bcl-2) and ubiquitination/degradation of p53 via the phosphoisitide/Akt pathway (O’Neill et al. 2012). Moreover, O-GlcNAc has also been implicated in the regulation of mitochondrial Bcl-2 levels following ischemia-reperfusion injury (Champattanachai et al. 2008). It is not surprising then that the inability to regulate these processes is incompatible with embryo viability.
3.9 Perturbed O-GlcNAcylation and Embryo Development
Whilst the capacity of the embryo to respond to stress by the HSP is essential to adaptation and survival, significant pertubations in O-GlcNAcylation arising from perturbed glucose flux through this pathway would have profound effects on development and may underlie the glucotoxic effects of hyperglycemia. Consistent with this hypothesis all treatments that perturb levels of O-GlcNAc either during oocyte maturation alone or through early development have a negative impact on early developmental outcomes. Increased activity of the HSP as a result of glucosamine supplementation during mouse and bovine oocyte IVM has detrimental effects on subsequent developmental competence (Sutton-McDowall et al. 2006; Schelbach et al. 2010). Moreover, pharmacological manipulation of the key enzymes of O-GlcNAcylation, OGT and OGA as well as nutrient manipulation of nucleoplasmic O-GlcNAc levels in early mouse embryos lead to decreased rates of development, reduced cellular proliferation and increased levels of apoptosis thus confirming that this system is critical for embryonic cellular homeostasis (Pantaleon et al. 2010).
Intriguingly, glucose deprivation does not cause a reduction in nucleoplasmic O-GlcNacylation as might have been anticipated given that there is no glucose flux through the HSP under these conditions. Indeed, levels of nucleoplasmic O-GlcNAc are increased significantly in response to embryo glucose deprivation (Pantaleon et al. 2010), consistent with data indicating that diverse forms of cellular stress which include heat stress, oxidative stress, ethanolic stress and osmotic stress all elevate the levels of O-GlcNAcylation (Zachara and Hart 2004). Inhibition of OGT using BADGP was not able to reverse this level of O-GlcNAcylation despite its efficacy on glucosamine-induced O-GlcNAc upregulation. This suggests that in the complete absence of glucose, and hence limited production of UDP-GlcNAc and reduced OGT activity, O-GlcNAcase activity may also be inhibited, highlighting the cooperative nature of the two enzymes acting in concert to maintain O-GlcNAcylation at levels required for cellular survival, hence the reason why diverse stress signals can act to increase levels of O-GlcNAc. However despite this apparent maintenance of O-GlcNAc levels in response to various stressors, continued absence of glucose during early development and thus disruption of cycling of O-GlcNAc on and off cellular proteins, is incompatible with cellular and embryo viability .
3.9.1 Embryotoxic Effects of Hyperglycemia and O-GlcNAcylation
Significantly, the toxicity associated with exposure of embryos to a hyperglycemic environment on a number of morphological parameters of early development including blastocyst formation (Fig. 3.2a), cell number and apoptosis is either reversed or ameliorated by inhibition of OGT (Pantaleon et al. 2010). This provides conclusive evidence that the HSP does indeed represent the mechanism by which hyperglycemia impacts adversely on early embryo development and survival. Previous studies had suggested that hyperglycemia triggers cell death in the early embryo by a decrease in glucose transporter GLUT1 expression, to trigger Bax-dependent apoptosis in the mouse blastocyst (Chi et al. 2000). This event however, is more likely to be secondary to dysregulation of the HSP and increased O-GlcNAcylation given the ability of OGT inhibition to reverse the impact of a hyperglycemic insult on embryo differentiation, proliferation and survival (Pantaleon et al. 2010).
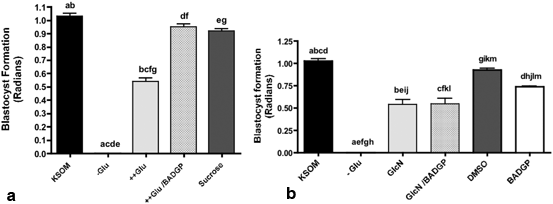

Fig. 3.2
a Effect of hyperglycemia in the presence and absence of the OGT inhibitor BADGP on mouse blastocyst formation. Zygotes were cultured from 18 h to 90 h post hCG in control KSOM, KSOM-glucose (-Glu) or KSOM- supplemented with 27 mM glucose (++Glu), or 27 mM glucose supplemented with 2 mM BADGP. 26.8 mM sucrose in KSOM (containing 0.2 mM glucose) was used as an osmotic control. Bars represent means ± SEM from three separate experiments each with 30 zygotes per treatment. Factorial ANOVA indicated no inter-experimental variation and no interaction (P> 0.05). Means with the same superscript are statistically different. (a–g, P< 0.001; Tukey’s post test). From (Pantaleon et al. 2010). b Effect of glucosamine (GlcN) in the presence of the OGT inhibitor BADGP on mouse blastocyst formation. Zygotes were cultured from 18 h to 90 h post hCG in control KSOM (0.2 mM glucose, KSOM), KSOM–glucose (-Glu), KSOM-glucose supplemented with 0.2 mM GlcN (GlcN), 0.2 mM GlcN in the presence of 2 mM BADGP and 5 mM GlcN in the presence of 2 mM BADGP. Bars represent means ± SEM from three separate experiments each with 30 zygotes per treatment. Factorial ANOVA indicated the lack of inter-experimental variation and no interaction (P > 0.05). Means were further analysed to determine differences between different treatments. Means with the same superscript are statistically different. (a–i, k: P < 0.01, j, l, m: P < 0.05, Tukey’s post test). From (Pantaleon et al. 2010)
The central mechanism implicated in the regulation of embryonic cellular proliferation and survival is the PI 3 kinase/Akt pathway (Riley et al. 2006). In the early embryo this survival signaling pathway is normally activated by trophic growth factor signals (Li et al. 2007) to reduce apoptosis and enhance cellular proliferation by activating downstream pathways of cellular growth, proliferation and survival. Additionally it regulates glucose transporter recycling at the plasma membrane allowing the embryo to co-ordinate nutrient transport and protein synthetic activities with the modified metabolic requirements of stimulated proliferation (Pantaleon and Kaye 1996). Impaired PI 3-kinase activation and reduced Akt activity in response to increased O-GlcNAcylation provides an explanation for decreased survival in response to hyperglycemia, defective glucose transporter recycling and stimulated cell proliferation and hence the insulin/growth factor resistance feature of diabetes (Whelan et al. 2010).
3.9.2 Glucosamine as a Hyperglycemic Mimetic
Whilst glucosamine can overcome the block to blastocyst formation (Pantaleon et al. 2008) in the absence of glucose and act as a potent stimulator of embryonic levels of O-GlcNAcylation (Pantaleon et al. 2010) it can be misleading as a hyperglycemic mimetic tool when used in isolation in vitro in the absence of glucose.
This is because in the absence of glucose, glucosamine may activate additional stress response pathways as a result of oxidative stress (Jansen et al. 2009), independently of effects through O-GlcNAcylation. Glucosamine enters cells and is readily phosphorylated to GlcN-6-P and thus enters the HSP downstream of entry into the PPP at F-6-P as discussed earlier (Fig. 3.1). In the absence of glucose, flux through the PPP is attenuated reducing NADPH production and increasing oxidative stress (Jansen et al. 2009). Moreover, GlcN-6-P is a potent inhibitor of the first enzyme of the PPP glucose-6-P-dehydrogenase (G6PDH)(Wu et al. 2001) and this high Glcn-6-P concentration arising from glucosamine supplementation may also limit PPP flux arising from any gluconeogenic activity, should this be occuring. The result is compromised development and increased cell death which cannot be reversed by OGT inhibition in sharp contrast to the ability of BADGP to reverse the glucotoxic effects of hyperglycemia on blastocyst formation completely (Fig. 3.2; Pantaleon et al. 2010).
Although such metabolism has not previously been reported in preimplantation embryos, administration of glucosamine to pregnant (day 7.5) mice inhibits the PPP and increases oxidative stress more potently than hyperglycemia in the whole embryo within 3 h (Horal et al. 2004). Moreover, recent studies in mouse oocytes aiming to establish dose response models for glucose and glucosamine in a mouse IVM system have demonstrated that glucosamine alone during IVM in the absence of glucose is unable to support subsequent embryo development (Frank et al. 2013) suggesting that the contribution of the PPP during oocyte maturation can be as critical for subsequent development as hexosamine signalling. Not only will this limit synthesis of NADPH (Wu et al. 2001) and thus increase oxidative stress but reduce the bioavailability of pentose phosphates required for nucleic acid synthesis to impact on embryo viability.
3.9.3 Periconceptional HSP Pertubation and Postnatal Outcomes
An in vivo mouse model of periconceptional glucosamine supplementation (daily glucosamine injections (20–400 mg/kg) administered for 3–6 days before and 1 day after mating) confirms the potential impact that perturbed HSP activity specifically during this developmental period can have on post natal outcomes. (Schelbach et al. 2013). Intriguingly there appeared to be an age dependency to different outcomes with reduced litter size independent of dose in younger mothers compared with a significantly higher incidence of fetal congenital abnormalities in older mothers, similar to and consistent with pathologies associated with hyperglycemic or diabetic mice (Schelbach et al. 2013). It is difficult to know the relevant contribution of effects on embryonic development versus maternal perturbations, given that maternal systemic glucosamine supplementation may also impact on decidualisation (Tsai et al. 2013) and by implication subsequent placentation to contribute to altered fetal growth in response to an adverse environment.
Nonetheless, the available evidence strongly implicates perturbed O-GlcNAc cycling as a result of either pharmacological inhibition or a perturbed nutrient environment with perturbed developmental outcomes, specifically the glucotoxic effects of hyperglycemia during preimplantation development . The system appears to be very tightly regulated since low and high levels of O-GlcNAcylation have a negative impact on early development with the potential to alter long-term health outcomes (Fig. 3.3). Based on the available evidence it is tempting to speculate that perturbed O-GlcNAcylation may underlie the impact of environmental stress associated with embryo culture that impacts on embryo viability and post-natal outcomes (Lane and Gardner 2005).
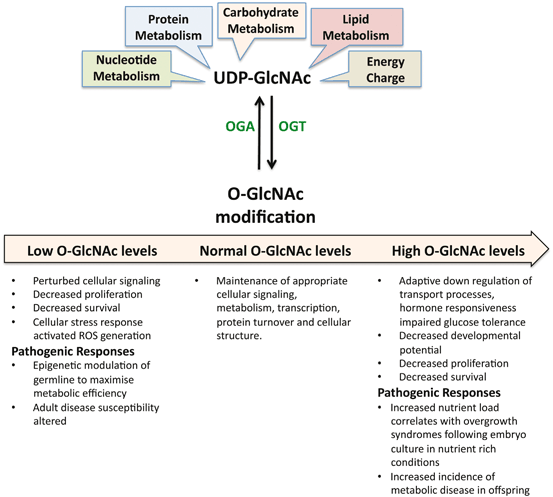

Fig. 3.3
Nutrient derived precursors integrated by the hexosamine biosynthetic pathway for the synthesis of UDP-GlcNAc. This activated sugar-nucleotide is used as the donor substrate for the O-GlcNAc modification of nucleoplasmic proteins by OGT. This modification is essential for cellular survival and embryo viability. Levels of O-GlcNAcylation are tightly regulated by the enzymes OGT and OGA. Inappropriately high and low levels of O-GlcNAc modification can have deleterious effects on early development with long term consequences as discussed in the text
3.10 Nutrient Stress, Embryonic Programming and O-Linked Glycosylation
Multiple stressors may alter growth trajectories through metabolic programming of the fetus and increase the chances of obesity, insulin resistance and cardiovascular disease later in life. The preimplantation period has been identified as particularly vulnerable to re-programming events which can ultimately lead to adult disease. Pregnant rodents fed a low protein diet (LPD) specifically during the preimplantation period give birth to animals that display signs of sustained hypertension (Kwong et al. 2000; Watkins et al. 2008) and anxiety-related behaviours that derive from a cellular memory established prior to implantation (Watkins et al. 2008). These outcomes are sexually dimorphic with male pups affected more adversely than females. Although the mechanisms involved in this early embryonic sensitivity and subsequent programming are elusive at least in the rat, the mothers are hyperglycaemic and hypoinsulinaemic (Kwong et al. 2000) raising the possibility that this embryonic phenotype may partly derive from perturbed maternal glucostasis and hence altered O-GlcNAcylation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


