The Reproductive System
The study of the endocrinology of the male and female reproductive systems is a specialized field and overlaps with urology, obstetrics and gynaecology. This chapter looks at some of the more common conditions that you are likely to encounter clinically and that have particular relevance to clinical biochemistry. Pregnancy and infertility are discussed in Chapter 10.
HYPOTHALAMIC-PITUITARY-GONADAL AXIS
The reproductive system is responsible not only for the production of hormones, but also for maturation of the germ cells in the gonads. It is important to understand the relationship between hormones with respect to these two functions if the results of reproductive endocrinology tests are to be interpreted correctly; see also Chapter 7.
Hypothalamic hormones
Anterior pituitary hormones
The gonadotrophins (LH and FSH), secreted by pituitary basophil cells, control the function and secretion of hormones by the testes and ovaries. The secretion of GnRH is pulsatile and thus so, in turn, is that of LH and FSH. Although there is only one releasing hormone, secretion of LH and FSH does not always occur in parallel and may be modified by feedback from the circulating concentrations of gonadal androgens or oestrogens. The actions of the gonadotrophins are:
LH primarily stimulates the production of hormones by the gonads,
FSH stimulates the development of the germ cells.
Gonadotrophin-releasing hormone analogues, for example goserelin, after an initial stimulation phase, down-regulate gonadotrophin secretion and have been used therapeutically for prostate carcinoma and endometriosis.
Prolactin, secreted by acidophil cells, is important during pregnancy and the post-partum period. It stimulates breast epithelial cell proliferation and induces milk production. Prolactin differs from all other pituitary hormones in its method of control. Secretion is inhibited, not stimulated, by dopamine (prolactin inhibitory factor); therefore, impairment of hypothalamic control causes hyperprolactinaemia. Its secretion is regulated by a short feedback loop between pituitary prolactin and hypothalamic dopamine via dopamine-2-type receptors.
Oestrogens stimulate the proliferation of pituitary lactotroph cells, although high oestrogen and progesterone concentrations inhibit secretion, as in pregnancy. Circulating prolactin concentrations are normally higher in pregnancy and increase during suckling as a result of the action of vasoactive intestinal peptide and also high oestrogen concentrations in pregnancy. Although thyrotrophin-releasing hormone (TRH) stimulates the secretion of prolactin, as well as of thyroid-stimulating hormone (TSH), this action does not seem to be of physiological importance; it may, however, be important in pathological conditions. Similar factors affect prolactin and growth hormone secretion. Secretion of both is pulsatile and increases during sleep and in response to physical and psychological stress.
Ovarian hormones
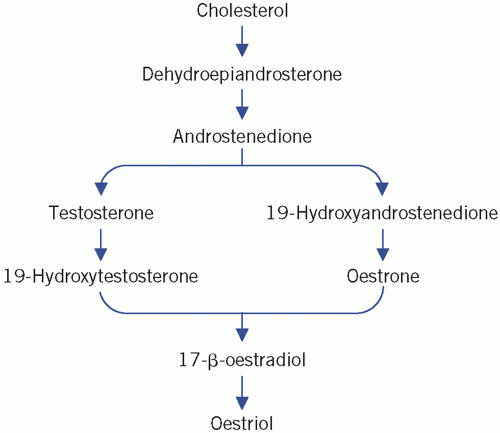
Oestrogens, progesterone and androgens are secreted by the ovarian follicles of the ovaries, which consist of germ cells (ova) surrounded by granulosa and theca cells. Androgens (C19 steroids), synthesized by theca
cells, are converted into oestrogens (C18 steroids) in the granulosa cells, a process that involves aromatization of the A ring and the loss of the C-19 methyl group (Fig. 9.1). Oestradiol is the most important ovarian oestrogen. The liver and subcutaneous fat convert ovarian and adrenal androgens to oestrone. Both oestradiol and oestrone are metabolized to the relatively inactive oestriol. Oestrogens are essential for the development of female secondary sex characteristics and for normal menstruation, and their concentration in plasma in children is very low. Androstenedione is the main androgen secreted by the ovaries. It is converted to oestrone and to the more active testosterone in extraovarian tissue. A small amount of testosterone is secreted directly by the ovaries. Plasma concentrations in women are about a tenth of those in men.
cells, are converted into oestrogens (C18 steroids) in the granulosa cells, a process that involves aromatization of the A ring and the loss of the C-19 methyl group (Fig. 9.1). Oestradiol is the most important ovarian oestrogen. The liver and subcutaneous fat convert ovarian and adrenal androgens to oestrone. Both oestradiol and oestrone are metabolized to the relatively inactive oestriol. Oestrogens are essential for the development of female secondary sex characteristics and for normal menstruation, and their concentration in plasma in children is very low. Androstenedione is the main androgen secreted by the ovaries. It is converted to oestrone and to the more active testosterone in extraovarian tissue. A small amount of testosterone is secreted directly by the ovaries. Plasma concentrations in women are about a tenth of those in men.
Progesterone is secreted by the corpus luteum during the luteal phase of the menstrual cycle and by the placenta. It prepares the endometrium of the uterus to receive a fertilized ovum and is necessary for the maintenance of early pregnancy. It also is pyrogenic and increases the basal body temperature. In plasma, only about 2 per cent of progesterone is unbound or free, the majority being bound to albumin and transcortin. Inhibin from granulosa cells inhibits FSH secretion while activin enhances the action of FSH and LH.
Testicular hormones
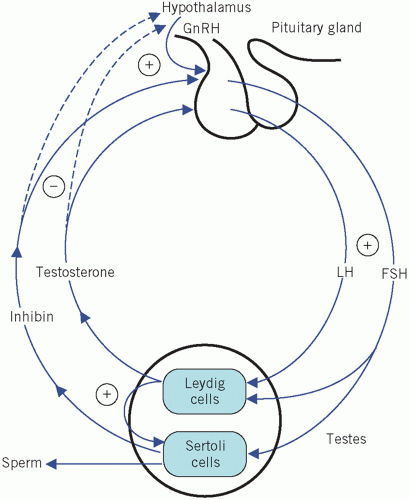
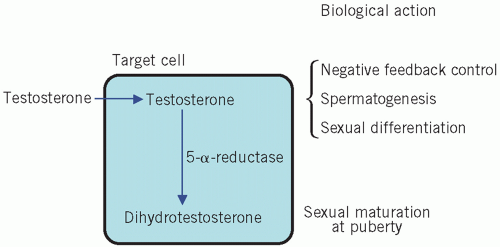
Testosterone is secreted by the Leydig cells, which lie in the interstitial tissue of the testes between the seminiferous tubules. The production of testosterone is stimulated by LH and it, in turn, inhibits LH secretion by negative feedback. Inhibin is a hormone produced by the Sertoli cells, part of the basement membrane of the seminiferous tubules, during germ cell differentiation and spermatogenesis. These processes require testosterone and are stimulated by FSH. Inhibin controls FSH secretion by negative feedback (Fig. 9.2) and activin enhances spermatogenesis. Testosterone is involved in sexual differentiation, the development of secondary sexual characteristics, spermatogenesis and anabolism. In the male, the effects of testosterone depend on intracellular conversion to the even more potent androgen dihydrotestosterone by the enzyme 5-α-reductase in target cells (Fig. 9.3).
Luteinizing hormone stimulates testosterone production from the Leydig cells. The Sertoli cells are involved in germ cell differentiation and spermatogenesis. These functions depend on testosterone and are stimulated by FSH.
Sex-hormone-binding globulin
Testosterone and, to a lesser extent, oestradiol circulate bound to a carrier protein, sex-hormone-binding globulin (SHBG), as well as to albumin. As with other hormones, only the free or unbound fraction (about 3 per cent of the total hormone concentration) is metabolically active. Plasma SHBG levels in females are about twice those in males. Changes in SHBG concentrations change the ratio of free testosterone to free oestrogen (see Table 9.1).
HYPERPROLACTINAEMIA
This is an important cause of amenorrhoea, sexual dysfunction, osteoporosis, infertility and possibly breast cancer. High plasma prolactin concentrations inhibit the normal pulsatile release of GnRH and inhibit gonadal steroid hormone synthesis directly. Plasma gonadotrophin and oestrogen concentrations are therefore low, and the symptoms of oestrogen deficiency may occur. About a third of patients with hyperprolactinaemia have galactorrhoea.
The finding of hyperprolactinaemia should be interpreted with caution. As mentioned above, plasma prolactin concentration is raised during pregnancy and lactation. Plasma prolactin concentrations are higher in females than in males and decrease post menopause. Samples for prolactin estimations should be taken at least 2-3 h after waking in order to eliminate the misleading elevated plasma concentrations found during sleep; the stress of venepuncture may also cause prolactin secretion. A sustained increase of more than about 700 mU/L should be investigated. Macroprolactinaemia, in which the raised plasma prolactin concentration is due to a complex with immunoglobulins, should be excluded, usually by being precipitated in the plasma sample by polyethylene glycol, before extensive investigation is undertaken for true hyperprolactinaemia.
CASE 1
A 34-year-old woman was seen in the endocrine clinic because of galactorrhoea and oligomenorrhoea. She was not taking any medication, and her renal function, liver function and blood glucose were normal. Her plasma biochemical results were as follows:
Thyroid-stimulating hormone 1.6 mU/L (0.20-5.0)
Free thyroxine 13.1 pmol/L (12-25)
Prolactin 2264 mU/L (<470)
Luteinizing hormone 7.2 U/L (1-25)
Follicle-stimulating hormone 4.4 U/L (1-15)
Testosterone 2.2 nmol/L (1-3)
Sex-hormone-binding globulin 58 nmol/L (20-90)
Oestradiol 544 pmol/L (70-880)
DISCUSSION
The patient has hyperprolactinaemia, with a common presentation. A pituitary magnetic resonance imaging scan was suggestive of a microprolactinoma (microadenoma). The other pituitary hormones are normal, presumably because the microprolactinoma had not encroached on the pituitary gland. Dopamine receptor agonists such as bromocriptine or cabergoline have been used to lower prolactin concentration under these circumstances.
Hypothyroidism, polycystic ovary syndrome,chronic kidney disease and certain drugs, e.g. antipsychotics, opioids, estrogens, H2 receptor antagonists and antidepressants, can also evoke hyperprolactinaemia. Antipsychotic drugs, such as chlorpromazine, haloperidol, clozapine, olanzapine and aripiprazole generally block dopamine receptors (D2), although the last may be associated with a low rate of hyperprolactinaemia. Prolactin plasma concentrations persistently greater than 1000 mU/L may need a change in antipsychotic medication or a reduction in the dose; concentrations over 2000 mU/L probably merit an endocrinologist’s opinion to exclude a prolactinoma.
The pathological causes of hyperprolactinaemia include a prolactin-secreting tumour of the pituitary gland. If a pituitary tumour is found, it is usually either a microadenoma (< 10 mm) or a macroadenoma (> 10 mm). The higher the plasma prolactin concentration, the greater the likelihood that a tumour is present, with concentrations more than 2000 mU/L
suggestive of hypothalamic or microadenoma, whereas concentrations more than 6000 mU/L are more likely to indicate a macroadenoma. The latter are sometimes associated with multiple endocrine neoplasia (MEN 1) syndrome (see Chapter 24).
suggestive of hypothalamic or microadenoma, whereas concentrations more than 6000 mU/L are more likely to indicate a macroadenoma. The latter are sometimes associated with multiple endocrine neoplasia (MEN 1) syndrome (see Chapter 24).
If hyperprolactinaemia is confirmed, pregnancy must be excluded, and also renal impairment, relevant drugs and hypothyroidism. Pituitary imaging with computerized tomography or magnetic resonance imaging may show a tumour. Dopamine receptor agonists such as bromocriptine or cabergoline are used to lower prolactin concentrations. Sometimes pituitary surgery is needed to remove a pituitary tumour.
The causes of hyperprolactinaemia are shown in Box 9.1 and a diagnostic algorithm in Figure 9.4.
SEXUAL DEVELOPMENT FROM CONCEPTION IN FEMALES AND MALES
The complex series of events leading to the development of sexual competence depends on many steps occurring at the correct time. The following simplified account aims to provide a background for the discussion of abnormalities of the system.
Box 9.1 Some causes of hyperprolactinaemia
Physiological, e.g. stress or pregnancy
Failure of hypothalamic inhibitory factors to reach the anterior pituitary gland due to:
Damage to the pituitary stalk by non-prolactinsecreting tumours of the pituitary gland or hypothalamus
Surgical section of the pituitary stalk
Other pituitary tumours
Microadenomas
Macroadenomas
Drugs
Estrogens
Dopaminergic antagonists, e.g.
Phenothiazines
Haloperidol
Metoclopramide
Methyldopa
Reserpine
Polycystic ovary syndrome
Chronic kidney disease (due to reduced plasma prolactin clearance)
Severe primary hypothyroidism (due to anterior pituitary stimulation by high thyrotrophin-releasing hormone concentrations).
Macroprolactinaemia
Chromosomal sex is determined at fertilization by the chromosomes present in the ovum and sperm, each of which contributes 22 autosomes and one sex chromosome, X or Y. Normal males have a 46,XY karyotype, and normal females 46,XX. Abnormalities
occurring at this stage may result in defective gonadal development, as occurs in Klinefelter’s syndrome in males (47,XXY) or Turner’s syndrome in females (45,XO).
occurring at this stage may result in defective gonadal development, as occurs in Klinefelter’s syndrome in males (47,XXY) or Turner’s syndrome in females (45,XO).
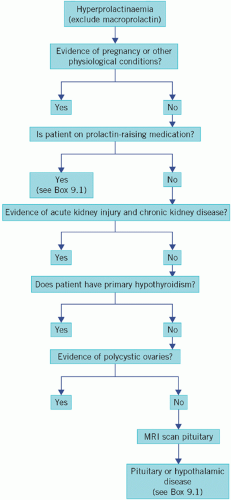 Figure 9.4 An algorithm for the investigation of hyperprolactinaemia. MRI, magnetic resonance imaging. |
The sex chromosomes determine whether the primitive gonads become testes or ovaries. The development and disorders of gonadal function in the male and female are considered separately below.
The female
Development of female characteristics
In the absence of a Y chromosome, the fetus starts to develop female characteristics at about 12 weeks of gestation. If androgens are produced at this stage, as, for example, in congenital adrenal hyperplasia (CAH), masculinization of the external genitalia may occur, causing female pseudohermaphroditism (see Chapter 8). Proliferation of fetal germ cells produces several million oocytes. By late fetal life, all the germ cells have degenerated and no more oocytes can be produced. Those oocytes that are present enter the first stage of meiosis and their numbers decline throughout the rest of the intrauterine period and childhood; the inability to replenish them explains the limit to the span of reproductive life in women, in contrast to the continuous ability of men to produce sperm. An abnormally high rate of decline leads to premature menopause.
Female puberty
At the onset of puberty, gonadotrophin secretion increases, as it does in the male. Ovarian oestrogen secretion rises and stimulates the development of female secondary sex characteristics and the onset of menstruation (menarche).
Normal gonadal function
At puberty the ovaries contain between 100 000 and 200 000 primordial follicles. During each menstrual cycle a small number develop, but usually only one reaches maturation, which is extruded from the ovary as an ovum (ovulation), and is ready for fertilization. The menstrual cycle is regulated by changing hormone concentrations (Fig. 9.5) and by changing sensitivity of ovarian tissue.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree