, Mohammed Al-Rubaie2, Stuart Walker3 and Sam Salek4
(1)
Regulatory Affairs Consultant Executive Directive, Kuwait Advancement for Conference and Exhibition Management, Kuwait, Kuwait
(2)
Ministry of Health, Directorate General of Pharmaceutical Affairs and Drug Control, Muscat, Oman
(3)
Founder of Centre for Innovation in Regulatory Science, London, UK
(4)
Department of Pharmacy School Life & Medical Sciences, University of Hertfordshire, Hatfield, UK
The availability of new medicines is influenced by different stakeholder perspectives including those of the regulators, policymakers and the pharmaceutical industry. These differences are reflected in the historical high-profile drug withdrawals and the rising demand for improving medicines development to protect patients from the predictable harms of adverse drug reactions. This issue has gained impetus in recent years due to societies in general becoming more risk averse. This is challenged by certain patient associations and advocacy groups for chronic or life-threatening conditions who would be willing to accept higher risks as a trade-off for improved benefits. Problems with the safety and efficacy profiles of medicines continue to be an issue despite the regulations that aim to maximise the benefit-risk balance of medicines before they are made available to patients. The pharmaceutical industry is under pressure to fulfil the regulatory requirements for marketing purposes, whilst regulators are being encouraged to reduce the registration requirements and to accelerate the approval of new medicines demanded by patients. This gives credence to the current initiative concerned with new adaptive pathways.
The Gulf Cooperation Council is currently facing a major regulatory dilemma (Fig. 12.1). This includes approvability, accessibility and affordability.
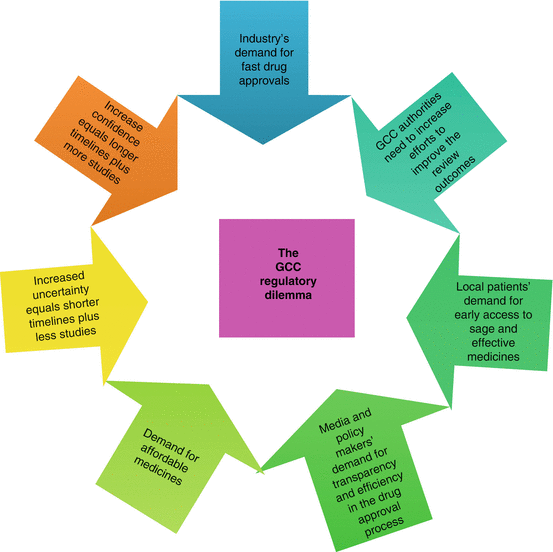

Fig. 12.1
The Gulf Cooperation Council (GCC) regulatory dilemma
Approvability – The GCC authorities need to increase their efforts to improve the regulatory review by enhancing their resources and training programmes, electronic management of processes and adopting a risk stratification model which would mean that the review (full review, abridged review and verification review) would be determined by where the product had previously been approved (reference agency).
Accessibility – Patients are continually demanding early access to safe and effective medicines that is in line with the industry’s demand for faster drug approvals. This situation is exacerbated by the delay that is seen between first world approval and the submission to the Gulf region. This is influenced by a number of factors including the regulatory requirements, the size of the market and the company strategy. These, of course, can be minimised by adopting an appropriate review model.
Affordability – The increasing cost of drug development means that modern medicines whilst being considered cost-effective can be expensive and as such cannot be paid for by limited healthcare budgets. This argues for the GCC now to implement appropriate health technology assessment rather than relying on reference pricing.
Centralised marketing authorisation is a process which facilitates the approval of a medicinal product from several regulatory authorities at one time, and this is carried out at a regional level. The centralised process has several benefits for the regulatory authorities and the pharmaceutical industry. It is intended to reduce the high costs incurred by pharmaceutical companies as they only have to submit a single application to obtain a centralised marketing authorisation valid in all GCC countries, and this leads to improved patient access. It facilitates the achievement of harmonised regulatory systems leading to quality and timely approvals, it allows for exchange of best regulatory review practices and it permits the marketing of new medicines that may compete with those already produced by domestic manufacturers. The Gulf states faced significant challenges in dealing with their established regulatory bodies, which were hesitant to give up their independence to the newly established GCC Central Drug Registration (GCC-DR) system (Al-Essa 2011). The idea of implementing a central registration system came originally from the industrialised world, especially from the success stories of the European Medicine Agency, which encouraged Gulf regulatory organisations to structure the central registration system.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


