Tests in the PPQ are based on the production batch record. Normally the product will be made according to the batch record (at target), however, during validation extra samples (both in time and quantity) need to be taken and stress conditions need to be used. Again, if development has documentation that the product can be run at various speeds, temperatures, mixing times, etc. then the PPQ is simpler. It is primarily run at target and one or two other conditions. These conditions should constitute the “worst” case conditions that one would expect to encounter during operation (eg, lowest speed and highest temperature). Worst case is not to make the product or production fail.
In demonstrating that the process is reproducible and in control (also see Chapter 10: Stage III—Collection and Evaluating Production Data), there are many items in the process pathway that need to be verified other than that it remains within the set control limits. For example, it may also include the impurities at different steps of the process (need to establish an impurity profile) and/or content uniformity. Items like this are general and may be applicable to most if not all products. Table 8.3 shows some measurable parameters (CQAs) for Tablets, Creams, Ointments and Liquid products.
Table 8.3
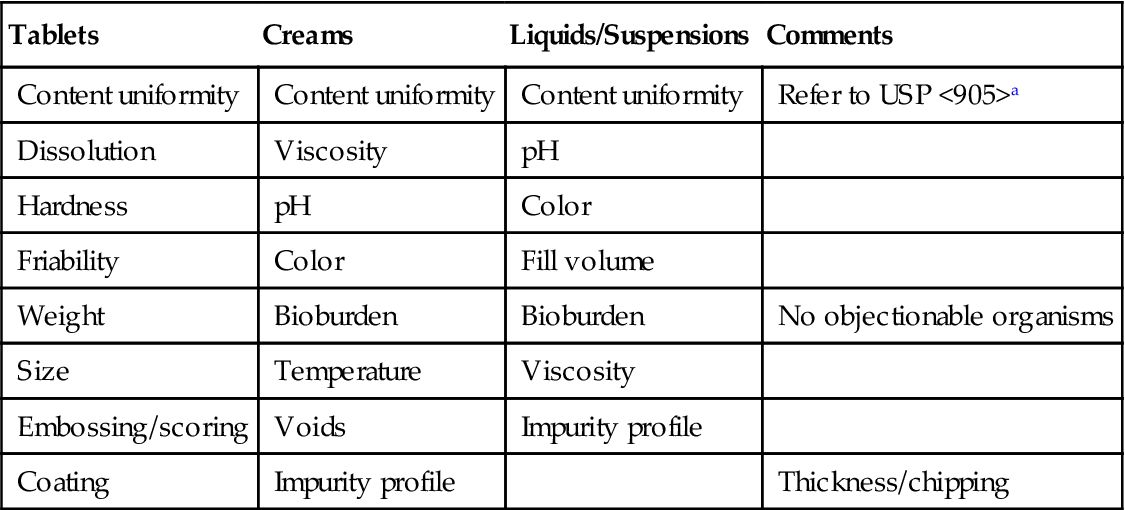
aUnited States Pharmacopeia; Uniformity of Dosage Units <905>, United States Pharmacopeial Convention, Inc.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree