(1)
Toyonaka, Japan
2.1 Introduction
From 1976 to 1977, three British groups established the acetylcholine (ACh) hypothesis, which claims that the activity of choline acetyltransferase (ChAT), an ACh synthase, is decreased in the brains of patients with Alzheimer’s disease (AD), causing a disorder in their cholinergic system with the decline in ACh (Bowen et al. 1976; Davies and Maloney 1976; Perry et al. 1977). Consequent to this development, researchers felt that replenishing ACh might treat AD. They attempted a treatment method involving supplementation of substances that serve as raw materials for ACh synthesis. ACh synthesis was expected to be catalyzed by ChAT from choline supplied from outside the brain and acetyl CoA produced inside the brain. Therefore, a clinical trial of choline administration using Lethicin was conducted. However, the efficacy of this treatment could not be confirmed. Next, a method was contrived to increase ACh in the synaptic cleft by inhibiting ACh esterase (AChE), an ACh-degrading enzyme (Fig. 2.1). Physostigmine, an alkaloid, shows AChE inhibitory activity. A clinical research study was also reported the use of tacrine as an AChE inhibitor (Summers et al. 1986). However, these drugs received poor clinical assessments. One reason was that physostigmine is an extremely unstable compound, and the other was tacrine-induced serious liver dysfunction.
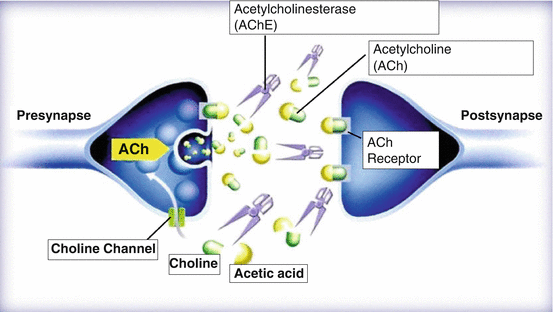

Fig. 2.1
Neurotransmission of cholinergic synapse. In the synaptic cleft of cholinergic neuron, acetylcholine (ACh) is decomposed into choline and acetic acid by acetylcholinesterase (AChE)
Hachiro Sugimoto of Eisai and his group also conducted drug discovery studies based on the ACh hypothesis. They used tacrine as a source for one compound. Unsurprisingly, all the synthesized derivatives showed strong toxicity, and none demonstrated any potential for commercialization. They also studied anti-hyperlipidemia drugs and discovered a certain compound that increased ACh, leading to the development of donepezil.
In Japan, phase I clinical testing of donepezil began in 1989. In the USA, the same testing began in 1991 and proceeded extremely smoothly. In November 1996, the US FDA approved donepezil for the use as an AD treatment drug. It is extremely rare for a drug to obtain approval only 8 months after application. Donepezil was approved in Europe in 1999 and received approval in Japan the same year.
2.1.1 ACh and Donepezil
According to the ACh hypothesis, a reduction in brain ACh levels is thought to be the cause of AD. AChE is an enzyme that breaks down ACh and makes it inactive. Donepezil impedes AChE and prevents the breakdown of ACh. This elevates the concentration of free ACh in the synaptic cleft, thereby activating cholinergic nerves and stopping the progression of cognitive failure mechanisms in AD patients (Fig. 2.2).
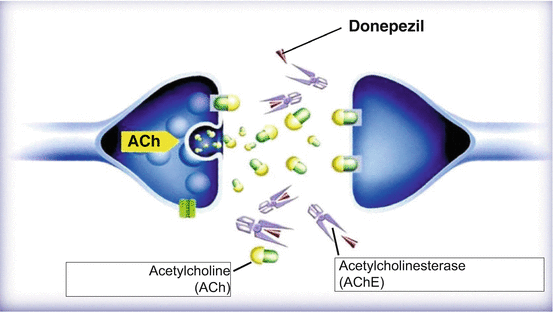

Fig. 2.2
Donepezil impedes AChE resulting in an increase of Ach level in the synaptic cleft
Butyrylcholinesterase (BuChE) is another in vivo cholinesterase. Whereas AChE is present mainly in neurons, BuChE is found in peripheral nerves and glia. It has low substrate specificity, and little is known about its physiological functions. Donepezil has an approximately 122-fold higher inhibitory effect on AChE than BuChE, as well as a high selectivity for AChE (Darvesh et al. 2003). To examine the selectivity of donepezil for central and peripheral nerves, researchers studied the minimum effective amount needed to elevate cerebral cortex ACh. They also studied the minimum effective amount to induce fasciculations, a peripheral nerve effect. The results showed that donepezil has a strong selectivity for cholinergic nerves (Kosasa et al. 1999). These facts likely explain why donepezil causes few peripheral adverse reactions. Donepezil has a long blood half-life, between 70 and 80 h, and can be administered once daily.
2.2 Effects Against AD
2.2.1 Effects on Cardinal Symptoms in Mild to Moderate AD
Donepezil was administered at 5 mg/day for 24 weeks to mild and moderate AD patients, and the ADAS-cog was used to evaluate cognitive function over time. Although almost no changes were seen in the placebo group, the ADAS-cog final score in the donepezil group dropped by 2.80 points, demonstrating an improvement in cognitive function (Homma et al. 2000).
A meta-analysis of donepezil randomized clinical studies conducted between 1986 and 2006 targeting dementia patients showed a significant improvement of cognitive function in AD patients (Raina et al. 2008).
The efficacy of long-term donepezil administration was also studied in mild and moderate AD patients. The results showed donepezil prevented a reduction in cognitive function for approximately 5 years (Roger et al. 2000).
2.2.2 Effects on Cardinal Symptoms in Advanced AD
Donepezil was administered at 5 or 10 mg/day to advanced AD patients (FAST stage: 6+; Mini-Mental State Examination (MMSE): 1–12 points). Those given 10 mg saw a significant improvement in the Clinician’s Interview-Based Impression of Change plus Caregiver Input (CIBIC-plus) and Severe Impairment Battery (SIB) scores (Homma et al. 2008).
2.2.3 Effects on the Biological and Psychological Symptoms of Dementia (BPSD) and on Caretaker Burden
The effects of donepezil on BPSD in mild and moderate AD patients were investigated using Neuropsychiatric Inventory (NPI) scores. The results showed improvement in BPSD (Holmes et al. 2004). A meta-analysis also revealed cholinesterase inhibitors, including donepezil, and showed improvement effects against BPSD (Trinh et al. 2003).
BPSD places a serious burden on caretakers. In one study, the time spent actually caring for a patient in his or her home was measured for a period of 1 year. The donepezil group saw nursing care time reduce by an hour each day, compared to the placebo group (Wimo et al. 2004). This result appears to be donepezil’s effect on BPSD.
2.2.4 Effects on Slowing Disease Progression
AD patients whose MMSE scores were ~20 prior to the release of donepezil saw their scores decrease by an average of approximately three points a year. Patients given donepezil showed a temporary increase in MMSE scores, followed by a decrease. However, the decrease was less than one point a year (Tomita et al. 2007).
Administration of donepezil was also shown to prolong the period patients could maintain ADL function by 72 %b (Mohs et al. 2001). This is reflected in data showing that those who took donepezil were able to delay placement in a nursing home by approximately 22 months compared to patients who did not take donepezil (Geldmacher et al. 2003).
As seen, donepezil is expected to affect disease progression. What sort of mechanism is at play? Neurotoxicity induced by glutamic acid administration is markedly suppressed by donepezil administered 24 h in advance, thus it appears to have neuroprotective effects (Takada et al. 2003). This suggests that donepezil is involved with nicotine receptors, in addition to its cholinesterase inhibition effects. Because it was reported that nicotine prevents glutamate neurotoxicity through nicotinic receptors (Akaike et al. 2010), donepezil was administered to mild and moderate AD patients for 24 weeks, and changes in hippocampal volume were measured before and after administration. The results revealed a significant difference between the two groups. Although a volume decrease was observed in the placebo group, no volume change was observed in the donepezil group (Krishman et al. 2003). There is data showing donepezil promoted neurogenesis in the hippocampus, to explain this effect (Kotani et al. 2006). Furthermore, although intraventricular injection of β-amyloid peptide (Aβ25–35) brings about lipoperoxidation, indicating hippocampal neurotoxicity, it is attenuated by donepezil administration (Meunier et al. 2006).
2.3 Effects Against Vascular Dementia
A double-blinded study in Europe and the USA reported efficacy against vascular dementia (VaD) (Roman et al. 2005). However, since it could not be ruled out that AD patients may have been included in the subject population, health insurance companies do not cover the use of donepezil for VaD (Maruki 2010). However, donepezil is recognized to be effective for the AD-VaD mixed type; therefore, health insurance companies cover its use in these patients (Rockwood et al. 2013).
2.4 Effects Against Dementia with Lewy Bodies
Dementia with Lewy bodies (DLB) is a disease characterized by Parkinsonism and dementia. However, many Parkinson’s disease (PD) patients present with both motor symptoms and cognitive dysfunction that progress slowly. Thus, DLB is also called PD dementia (PDD). Until now, PDD and DLB were differentiated according to the order in which Parkinsonism and dementia develop. However, since both diseases have Lewy bodies as a pathologic common finding, we will handle both DLB and PDD (which includes PD) in this chapter by referring to them collectively as Lewy body disease.
2.4.1 Intracerebral ACh Nerves
ACh nerves, which are central nerves, can be broadly classified into three systems. ACh-ergic interneurons are present inside the striate body. These interneurons are in close contact with dopaminergic (DA-ergic) neuron endings, regulating the DA-ACh balance (Aosaki et al. 2010). In PD, DA neuron endings projecting from the substantia nigra pars compacta are progressively lost. Because of this, ACh becomes predominant inside the striatum, relatively speaking, and disrupts the DA-ACh balance. Anticholinergic drugs are effective against PD motor symptoms, including tremor.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


