Fig. 8.1
Structure of capsaicin. Capsaicin has three main moieties: vannillyl (methylcatechol group), amide bond, and an alkyl side chain
Capsaicin is absorbed very rapidly by a nonactive process from the stomach and whole intestine with a maximum blood concentration observed at 1 h after administration (Suresh and Srinivasan 2010). Capsaicin is mainly metabolized in the liver through cytochrome P450-dependent reactions and peroxydase enzymes, producing three major metabolites, 16-hydroxycapsaicin, 17-hydroxycapsaicin, and 16, 17-hydroxycapsaicin, and is scarcely excreted in the urine (Suresh and Srinivasan 2010). Although many enzymes may play some role in hepatic drug metabolism, cytochrome P450 enzymes are quantitatively the most important, and it has been proposed that many drug–drug interactions result from the alteration (increase or decrease) in the activities of these enzymes (Zhang et al. 2012; Zhai et al. 2013). However, Babbar et al. demonstrated that at concentrations occurring after ingestion of chili peppers or topical administration of a high-concentration patch, capsaicin did not cause direct inhibition of any CYP enzyme (Babbar et al. 2010). Capsaicin toxicity is minimal at low doses. Previous research on the metabolism of capsaicin has proposed the formation of potentially deleterious reactive intermediates. It has been suggested that capsaicin is converted into a mutagenic or carcinogenic form through metabolism, such as an electrophilic epoxide or quinone derivatives (Surh and Lee 1995). However, research by Reilly et al. (2003) support the hypothesis that P450-mediated metabolism of capsaicin represents a detoxification mechanism by decreasing its pharmacological and/or toxicological activity and may serve to ameliorate the effects of capsaicin by preventing key biochemical interactions with molecular determinants of toxicity (Reilly et al. 2003). It must be taken into account that the metabolism of capsaicin by different P450 enzymes expressed in different tissues may yield different reactive intermediates in different cell types, which may partially explain conflicting reports related to the cytotoxic, procarcinogenic, and chemoprotective effects of capsaicinoids in different cells and/or organ systems (Reilly et al. 2013).
8.2 TRPV1 Receptor
It has been well documented that capsaicin targets the receptor TRPV1, first cloned from rat (Caterina et al. 1997) (see Chap. 2 of this book). TRPV1 is a cation channel belonging to the superfamily of transient potential receptors (TRPs) which are subdivided into seven subfamilies based on their amino acid sequence homology: TRPC (canonical), TRPV (vanilloid), TRPA1 (the only member of the subfamily ankyrin), TRPM (melastatin or long TRPs), TRPP (polycystin), TRPN (nonmechanoreceptor potential C), and TRPML (mucolipin). The vanilloid TRP subfamily comprises six members (TRPV1-6). Based on sequence homology and functional similarity, the six TRPV channels are divided into nonselective cation channels group 1 (TRPV1, TRPV2, TRPV3, and TRPV4), and selective Ca2+ channels group 2 (TRPV5 and TRPV6). TRPV1 is a nonselective cation channel but prefers Ca2+ over Na+. Until now, capsaicin has been identified uniquely as an agonist at TRPV1.
Structurally, a functional TRPV1 channel is a tetrameric membrane protein with four identical subunits assembled around a central aqueous pore which forms the cation channel. Each subunit harbors six membrane-spanning domains with a short, pore-forming hydrophobic loop between the fifth and sixth transmembrane domains, an N-terminal domain with six ankyrin repeats (ARD), and a C-terminal domain residue within the cytoplasm (Fig. 8.2). Capsaicin binds to the TRPV1 protein channel in the intracellular S2–S4 region with high affinity mediated by interactions between the vanilloid group of capsaicin and the benzene ring of aromatic residues (Jordt and Julius 2002). The ARD of the N-terminal region contains an ATP-binding as well as a Ca-calmodulin binding site (Lishko et al. 2007). Phosphatidylinositol-4,5-bisphosphate (PIP2) binds to the C-terminus of the receptor and inhibits channel gating (Prescott and Julius 2003). Either ATP or PIP2 prevent desensitization to repeated applications of capsaicin whereas calmodulin has the opposite effect (Lishko et al. 2007).
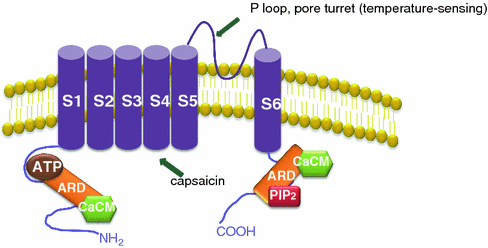

Fig. 8.2
The TRPV1 receptor. TRPV1 channel is a tetrameric membrane protein with four identical subunits. Each subunit harbors six membrane-spanning domains with a short, pore-forming hydrophobic loop between the fifth and sixth transmembrane domains, an N-terminal domain with six ankyrin repeats (ARD), and a C-terminal domain residue within the cytoplasm. The ARD of the N-terminal region contains an ATP-binding as well as a Ca-calmodulin binding site
Besides by capsaicin, TRPV1 receptor is activated by heat, low pH, and other ligands, leading to a burning pain sensation. Recent findings suggest that heat and capsaicin activate TRPV1 at different sites and they may act synergistically to stimulate channel gating (Cui et al. 2012).
The expression of TRPV1 has been demonstrated in most of the tumor cells analyzed. TRPV1 is expressed in human breast cancer MCF-7 and BT-20 cells (Chang et al. 2011; Vercelli et al. 2013) as well as in canine mammary cancer cells (Vercelli et al. 2013). Prostate cancer derived LNCaP and PC-3 cells as well as Benign Prostate Hyperplasia (BPH) tissue expresses TRPV1 (Sanchez et al. 2005). In those cells, capsaicin evokes an increase in the intracellular calcium concentration which is blocked by the TRPV1 antagonist capsazepine, implying a functional receptor (Sanchez et al. 2005). TRPV1 ion channels are expressed in glioma cell lines and in glioma tissues as well as in several tumor cells of astrocytic origin, and in normal human astrocytes (Biggs et al. 2007).
Changes in the expression of TRPV1 are often associated with changes in tumor progression and prognosis. Clinical investigation found a remarkably elevated TRPV1 expression in the epithelial cells of prostate cancer samples when compared to either the healthy or the BPH sections (Czifra et al. 2009). The expression of TRPV1 increased in parallel and gradually with the Gleason grade (Czifra et al. 2009). High TRPV1 expression in tumorigenic cell lines may play roles in the development and progression of hepatocellular carcinoma and metastatic liver cancers (Rychkov and Barritt 2011). By contrary, a decrease of TRPV1 expression can occur during the development of human urothelial cell carcinoma (UCC). Levels of TRPV1 are strongly reduced in high-grade and stage invasive of transitional cell carcinoma of human bladder and low TRPV1 expression is related with poor prognosis (Santoni et al. 2012). In line with this, it has been proposed that chronic blockade of TRPV1 might increase the risk of tumor development. Topical administration of a TRPV1 antagonist in mice promoted mouse skin tumorigenesis (Li et al. 2011). Likewise, capsaicin treatment induced a more aggressive gene phenotype and invasiveness in null-TRPV1 urothelial cancer cells (Caprodossi et al. 2011).
However, as described below, in most studies performed, capsaicin-induced tumor cell growth inhibition and apoptosis is independent of its stimulatory effects on the TRPV1 receptor, because co-treatment with competitive vanilloid receptor inhibitors like capsazepin did not protect the cells from capsaicin-induced death (Kim et al. 2004; Athanasiou et al. 2007; Zhang et al. 2008); moreover, it increases the cytotoxic effect of capsaicin (Sanchez et al. 2006; Sung et al. 2012).
8.3 Antitumor Properties of Capsaicin in Different Cancer Cells
Cancer is caused by defects in the mechanisms underlying cell proliferation and cell death. The development of tumors results from excessive cell proliferation combined with inhibition of cell apoptosis, which eventually leads to imbalances in tissue homeostasis and uncontrolled proliferation. Hence, targeting cell proliferation or induction of cancer cell apoptosis is one of the major challenges in cancer research. Capsaicin has been shown to inhibit cell proliferation, to induce cell cycle arrest and to trigger apoptosis in many cancer cell types (summarized in Table 8.1).
Table 8.1
Cancer cell lines sensitive to capsaicin-induced cell death
Effective doses (μM) | IC50 (μM) | References | |
|---|---|---|---|
Colorectal cancer | |||
LoVo | 100–200 | 100 | Lee et al. (2012) |
HCT-116 | 50–250 | 250, 25 | |
SW480 | |||
COLO 205 | 25–500 | 150 | Lu et al. (2010) |
Prostate cancer | |||
PC-3 | 5–50 | 20 | |
DU-145 | 0.01–500 | 10 | Mori et al. (2006) |
LNCaP | 0.01–500 | 200 | Mori et al. (2006) |
Breast cancer | |||
MCF-7, SKBR-3, T47D, BT-474 | 1–200 | 50, 200, 300 | |
MDA-MB-231 | 200, 22 | ||
Urinary bladder | |||
T24 | 50–200 | 150 | Yang et al. (2010) |
Myeloid leukemia | |||
NB4, Kasumi-1 | 10–200 | 50 | Ito et al. (2004) |
UF-1, HL60 | 50–1,000 | 80, 150 | |
U937 | 50–250 | 500, 200 | Moon et al. (2012) |
HPB-ATL-T, HPB-CTL-I, HUT-102 | 50–200 | Zhang et al. (2003) | |
HPB-ALL, Jurkat T | 100 | ||
Gastric carcinoma | |||
SCM1 | 0.1–200 | 200 | Wang et al. (2008) |
Hepatocellular carcinoma | |||
HepG2 | 10–200 | 75, 200, >200 | |
Pancreatic cáncer | |||
PANC-1 | 50–300 | 200 | Zhang et al. (2013) |
AsPC-1 and BxPC-3 | 25–250 | 150 | Zhang et al. (2008) |
Cutaneous squamous cell carcinoma | |||
COLO 16 | 25–200 | 100 | Hail and Lotan (2002) |
Melanoma | |||
A375 | Kim (2012) | ||
Nasopharyngeal carcinoma | |||
NPC, NPC-TW 039 | 200–400 | 250 | Ip et al. (2012) |
Small cell lung cancer | |||
H69 | 1–100 | 50 | Brown et al. (2010) |
Others | |||
KB cells | 50–250 | 150 | Lin et al. (2013) |
8.3.1 Colorectal Cancer
Colorectal cancer (CRC) is one of the most lethal cancers worldwide and the third leading cause of cancer-related death in the United States (Siegel et al. 2012; Quaglia et al. 2013). It is estimated that there are nearly 1.2 million men and women living in the United States with a previous diagnosis of colorectal cancer, and an additional ~150,000 will be diagnosed each year (Siegel et al. 2012). The poor prognostic outcome of colorectal cancer is due to its resistance to current therapies. Although about half of individuals with CRC could be cured by surgery, radiation therapy, and/or chemotherapy treatment before tumor cell dissemination, 40–50 % of patients had metastatic diseases and prognosis is poor with a 5-year survival <10 % (Quaglia et al. 2013).
Recent findings suggest that deregulation of the mucosal immune system predisposes to inflammatory bowel disease (IBD) and CRC. The role of inflammation in carcinogenesis was first proposed by R. Wirchow in the nineteenth century after observations in tumor samples which were infiltrated by high numbers of leukocytes (Bochalli 1952). A tumorigenic niche, composed of inflammatory cells, myofibroblasts, other cell types, and extracellular components, is essential for the maintenance of malignancies and facilitates tumor initiation, progression, and survival. Indeed, human colon cancer tissues are infiltrated by inflammatory cells and plasma levels of cytokines are often raised in CRC patients (Vaiopoulos et al. 2013). Regulation of the inflammatory response is very complicated but many signaling pathways involve the transcription factor NFκB. Capsaicin downregulates NFκB (see below) behaving as a very potent inhibitor of inflammation (revised in this book) and hence may contribute to prevent CRC. Besides this, capsaicin functions as an antitumoral drug as it inhibits the growth and proliferation of different CRC cell lines. High-concentration capsaicin (≥200 μM for SW480 and CT-26 cell lines; ≥25 μM for HCT116 cell line) showed antiproliferative activity in CRC cells in a dose-dependent manner (Yang et al. 2013). In another study, an inhibition of cell growth and transcriptional activity was observed at 100 μM (Lee et al. 2012) (Table 8.1).
8.3.2 Pancreatic Cancer
Pancreatic cancer, one of the most fatal types of solid malignancy, is the fourth leading cause of cancer-related mortality in the USA and other industrialized countries. Despite efforts over the past three decades to improve diagnosis and treatment, the prognosis for patients with pancreatic cancer is extremely poor with or without treatment, and incidence rates are virtually identical to mortality rates (Tamburrino et al. 2013). Srivastava et al. demonstrated that capsaicin-triggered apoptosis in human pancreatic cancer cells, which represent one of the most difficult types of cancer to treat. Capsaicin increased the number of apoptotic proteins and increased cytochrome c levels in the cytosol in pancreatic AsPC-1 and BxPC-3 cells. Capsaicin-induced apoptosis in pancreatic cancer cells was associated with the generation of reactive oxygen species (ROS) and persistent disruption of mitochondrial transmembrane potential without affecting the normal cells (Zhang et al. 2008; Pramanik et al. 2011). Likewise, in pancreatic cancer PANC-1 cells, capsaicin dose-dependently induced apoptosis, via downregulation of the PI3 K/Akt pathway (Zhang et al. 2013).
8.3.3 Hepatocellular Carcinoma
Hepatocellular carcinoma is the most common form of liver cancer, and its incidence has been increasing worldwide, representing 70–85 % of primary hepatic malignancies in adults. Liver transplant is the first choice of treatment because it eliminates malignant cells and restores liver function. However, unfortunately 20 % of patients suffer tumor recurrence and in spite of intense research on HCC treatment, no relevant improvement in the survival of patients with HCC has been achieved so far (Llovet and Bruix 2008). Therefore it is urgent to identify novel therapeutic strategies for the management of HCC.
Capsaicin induced apoptosis in the hepatocellular carcinoma cell line HepG2 with the involvement of intracellular Ca2+, ROS, Bcl-2 family, cytochrome c protein expression, and caspase-3 activity (Huang et al. 2009). In the same cells, capsaicin increased ROS production and induced expression of heme oxygenase-1 (HO-1) (Lee et al. 2004; Joung et al. 2007). Likewise, capsaicin sensitized hepatocellular carcinoma cells as well as breast cancer cells and leukemia cells to apoptosis induced by tumor necrosis factor-related, apoptosis-inducing ligand (TRAIL) (Moon et al. 2012). Co-treatment with capsaicin and TRAIL potentiated the caspase 3, 8, and 9 activation, the release of cytochrome C, and the disruption of mitochondrial transmembrane potential.
8.3.4 Prostate Cancer
Prostate cancer is one of the most prevalent forms of cancer in men worldwide. It is estimated that there are nearly 2.8 million men living with a history of prostate cancer in the United States, and an additional 241,740 cases have been diagnosed in 2012. Although prostate-specific antigen (PSA)-based screening has substantially reduced the incidence of mortality from prostate cancer (Carter et al. 2013; Vickers et al. 2013), it has increased the number of new cases diagnosed. Therefore, the increasing prevalence and the complexity of cancer treatment techniques require a great effort to avoid negative treatment effectiveness. Treatment options vary depending on the stage and grade of the cancer, as well as patient comorbidity, age, and personal preferences. More than one-half (57 %) of men aged younger than 65 years are treated with radical prostatectomy. Those aged 65–74 years commonly undergo radiation therapy (42 %), although radical prostatectomy (33 %) is also often used. Androgen deprivation therapy, chemotherapy, bone-directed therapy (such as zoledronic acid or denosumab), radiation therapy, or a combination of these treatments are used to treat more advanced disease. Unfortunately, too often the appearance of hormone refractory cancer cells leads eventually to the recurrence of cancer which turns to a hormone-independent state for which there is not treatment. The development of such lethal, castration-resistant prostate cancer (CRPC) is associated with adaptive changes to the androgen receptor (AR), including the emergence of mutant receptors and truncated, constitutively active AR variants.
Scientific progress in prostate cancer treatment has often been based on the use of established cell lines which mimics different prostate cancer stages. The LNCaP cell line is derived from a biopsy of a lymph node of a 50-year-old Caucasian male with confirmed diagnosis of metastatic prostate carcinoma (Horoszewicz et al. 1983). These cells are responsive to classic AR agonists and therefore are used as a model for androgen-sensitive prostate cancer. It has been shown that in the LNCaP cells high doses of capsaicin downregulates the expression of the AR (Mori et al. 2006). AR regulates the transcription of the PSA gene, as the 5′ upstream promoter and enhancer region of the PSA gene has several androgen response elements (ARE). Capsaicin at high doses inhibits the ability of dihydrotestosterone to activate the PSA promoter/enhancer even in the presence of exogenous AR which suggests that capsaicin inhibits the transcription of PSA not only via downregulation of expression of AR, but also by a direct inhibitory effect on PSA transcription (Mori et al. 2006). Those effects are TRPV1 receptor independent since the antagonists, capsazepine and ruthenium red, do not have any effect (Mori et al. 2006). Moreover, doses used to induce apoptosis on LNCaP prostate cells are very high (200 μM) and far away from the Kd of the receptor. However, capsaicin at lower doses (10–20 μM) induces upregulation of androgen receptor expression in LNCaP cells which is prevented by the TRPV1 antagonist capsazepine (Malagarie-Cazenave et al. 2009).
PC-3 cell line was originally derived from advanced (grade IV) androgen-independent prostate adenocarcinoma metastasized to bone (Iype et al. 1998). They are tumorigenic and have been widely used as a model for androgen-resistant prostate cancer. DU-145 cells were derived from a human prostate adenocarcinoma metastatic to the brain and are also used to study the androgen-resistant prostate cancer (Stone et al. 1978). In this androgen-independent cell lines, capsaicin dose-dependently induces cell cycle arrest and apoptosis (Mori et al. 2006; Sanchez et al. 2006, 2007, 2008). The mechanisms underlying vanilloid-induced apoptosis seem to occur independently of the TRPV1 receptor. In fact the apoptotic effect elicited by capsaicin in PC-3 cells cannot be reversed by the TRPV1 antagonist capsazepine, which in turn exerts cytotoxic effect on prostate cells (Mori et al. 2006; Sanchez et al. 2006). The androgen-resistant cell lines are more sensitive to capsaicin-induced cell death as doses of capsaicin required to induce cell death are lower than that required in the LNCaP cells (Table 8.1).
8.3.5 Breast Cancer
Breast cancer is the second leading cause of cancer death in women, exceeded only by lung cancer. It is estimated that 1 out of 10 women will develop breast cancer during her life and that there are nearly 3 million women living in the United States with a history of invasive breast cancer (Siegel et al. 2012). Although significant advances have been made in the treatment of this cancer, only less than half of the patients treated for localized breast cancer benefit from adjuvant chemotherapy; and most patients with metastatic cancer eventually develop disease that is resistant to therapy. In addition to stage, factors that influence survival include tumor grade, hormone receptor status, and human epidermal growth factor receptor 2 (HER2) status.
Several reports indicate that capsaicin inhibits the growth of breast cancer cell lines. Inhibition of growth is associated with cell cycle arrest in the S-phase and increased levels of apoptosis both in caspase-3-deficient MCF-7 cells and in non-caspase-3-deficient BT-20 cells suggesting that apoptosis induction is caspase-3 independent (Chou et al. 2009; Thoennissen et al. 2010; Chang et al. 2011).
Notably, in breast cancer cell lines, capsaicin was able to decrease the protein levels of both HER-2 and EGFR (Thoennissen et al. 2010). In HER-2 overexpressing BT-474 and SKBR-3 cell lines, which only have low to moderate levels of EGFR, capsaicin was able to clearly reduce expression levels of both types of receptors. In contrast, the drug showed less activity in the MDA-MB231 cell line, which is known to overexpress HER-2 as well as EGFR. However, capsaicin did not alter the mRNA levels of EGFR and HER-2 in all three breast cancer cell lines, suggesting that capsaicin decreases the two receptors by affecting their protein stability (Thoennissen et al. 2010).
In another study performed by Yoon et al. capsaicin treatment of MCF-7 cells induced S-phase arrest and the accumulation of p53 in the nucleus and cytosol. Capsaicin also induced autophagy through the AMPKα-mTOR signaling pathway which was further confirmed by Atg5 induction, LC3 conversion, and decreased p62 in a dose-dependent manner (Yoon et al. 2012).
Likewise, Dou et al. showed that whole pepper extracts were effective in inhibiting cell growth and inducing apoptosis in breast cancer cell lines MDA-MB-231 and MCF-7 and in Jurkat T leukemia cells. The peppers used in this study ranged in capsaicin levels from the bell pepper (0 Scoville heat units) to the habanero chili (100,000–350,000 Scoville heat units) and they were compared with purified capsaicin (16,000,000 units) (Dou et al. 2011). The effectiveness of the pepper extracts was correlated with the levels of capsaicin present according to the Scoville scale. Both pepper extracts and purified capsaicin induced apoptosis and decreased VEGF secretion in breast cancer cells whereas has minimal effect in breast normal cells (Dou et al. 2011). The mechanism whereby the extracts and capsaicin induced apoptosis was not related to the presence/absence of estrogen receptors since both cell lines differ in the ER expression (MCF-7 are estrogen receptor-positive and MDA-MB-231 are estrogen receptor-negative) (Thoennissen et al. 2010). However, it was reduced by the hydroxyl radical scavenger thiourea, suggesting the involvement of reactive oxygen species in the apoptotic effect.
8.3.6 Leukemic Cells
Capsaicin suppressed the growth of leukemic cells, but not normal bone marrow mononuclear cells, via induction of G0–G1 phase cell cycle arrest and apoptosis. Capsaicin-induced apoptosis was in association with the elevation of intracellular reactive oxygen species and Ca2+ production accompanying a downregulation of mitochondrial membrane potential (Δψm) (Ito et al. 2004; Tsou et al. 2006). Interestingly, capsaicin-sensitive leukemic cells were possessed of wild-type p53, resulting in the phosphorylation of p53 at the Ser-15 residue by the treatment of capsaicin which was abrogated by pre-treatment with the antioxidant NAC (Ito et al. 2004). In human myeloid leukemia cells capsaicin binds to prohibitin (PHB) 2, which is normally localized to the inner mitochondrial membrane, and induces its translocation to the nucleus thereby causing apoptosis. In human acute monocytic leukemia cells THP-1, capsaicin increases the gene expression of carnitine palmitoyltransferase 1 (CPT-1) and the oxidation rate of palmitate. This data suggests that capsaicin-induced attenuation of palmitate-induced inflammatory gene expression is associated with reduced activation of JNK, c-Jun, and p38 and improved β-oxidation (Choi et al. 2011).
The approach of treating cancer with combinations of standard chemotherapeutic drugs has become increasingly common. In line with this, studies performed by Schwartz et el. demonstrated that the addition of capsaicin to a combination of molecules targeting the cancer metabolism markedly decreased lung or bladder carcinoma and melanoma tumor cell growth in mice (Schwartz et al. 2013).
8.3.7 Lung and Bronchus
Lung cancer is a major cause of morbidity and mortality worldwide in both men and women, accounting for 29 % of all cancers. The median age at diagnosis for lung cancer is 70 years for males and 71 years for females. The majority of lung cancers (56 %) are diagnosed at a distant stage because early disease is typically asymptomatic; only 15 % of cases are diagnosed at a local stage (Matsuda and Machii 2013). Lung cancer is classified as small cell (14 % of cases) or non-small cell (85 % of cases) for the purposes of treatment. Radiation therapy alone (for limited disease) or combined with chemotherapy (for extensive disease) is the standard treatment for small cell lung cancer. Although it has been reported the chemopreventive effect of capsaicin in experimental models of lung carcinogenesis, there are few studies showing the antitumor activity in lung cancer. In four human small cell lung cancer (SCLC) cells, capsaicin displays potent antiproliferative activity with the contribution of the E2F family of transcription factors which mediate cell cycle arrest (Brown et al. 2010).
8.4 Capsaicin Cancer-Promoter Activity
Despite our increasing understanding of the anticancer effects of capsaicin on the above-mentioned cancer cell lines, conflicting data from animal models and basic research suggest that in some circumstances capsaicin may promote tumor growth.
Low doses of capsaicin may exert protumorigenic effects in several cancer cell lines. Human colorectal carcinoma cell line SW480 and HCT116, and murine colorectal carcinoma CT-26 cell line revealed a 4-fold elevation in cell motility upon treatment with 12.5 μM capsaicin, which had no impact on cell proliferation (Yang et al. 2013). Likewise, HCT116 human colon carcinoma cells treatment with low concentrations of capsaicin (≤10 μM) enhanced cell proliferation and migration in association with upregulation of ENOX2 (tumor-associated NADH oxidase) expression (Liu et al. 2012). The same group has demonstrated that higher doses of capsaicin (250 μM) inhibit HCT116 cell proliferation and downregulate ENOX2 (Mao et al. 2008). In human keratinocytes from perilesional vitiligo skin, capsaicin used at a concentration of 10 μM, induced an increase in mitochondrial activity and reduced ROS accumulation and NFκB and p53 activation (Becatti et al. 2010).
In the same line, the group of Yang et al. demonstrated that low doses of capsaicin (12.5 μM) enhanced both migratory and invasive capability of HCT116 cell line whereas higher doses (200 μM) induced cell cycle arrest and inhibit cell proliferation (Yang et al. 2013). Furthermore, 100 μM capsaicin induced epithelial-to-mesenchymal transition, upregulated expression of MMP-2 and MMP-9, and activated Akt/mTOR and STAT-3 pathways in colorectal cancer cells (Yang et al. 2013). We have observed that capsaicin at lower doses (10–20 μM) induces cell proliferation and upregulation of the androgen receptor expression in prostate cancer LNCaP cells (Malagarie-Cazenave et al. 2009). Interestingly, this effect was TRPV1 receptor-dependent since it was prevented by the TRPV1 receptor antagonist capsazepine (Malagarie-Cazenave et al. 2009).
The biphasic effect of capsaicin observed in many tumor cells might be due to the activation of TRPV1. As stated above, most tumor cell lines expressed TRPV1 which has an affinity for capsaicin of Kd = 710 nM. Low doses of capsaicin (≤20 μM) were effective in inducing calcium entry through TRPV1 receptor in cultured cells (Vriens et al. 2004; Sanchez et al. 2005). In line with this, it has been observed that Ca2+ entry via a capsaicin-sensitive membrane channel may play a role in stimulating hepatocellular carcinoma HepG2 cells cell migration (Vriens et al. 2004). Therefore at low doses capsaicin presumably activates TRPV1 which could influence intracellular calcium levels affecting cancer cell aggressiveness. However, the capsaicin-induced apoptotic effect at high doses is a receptor-independent mechanism, as observed in most cancer cell lines. In addition, recent findings demonstrate that in TRPV1 null cells, capsaicin exhibits a more aggressive antiproliferative effect than in TRPV1 expressing cells (Caprodossi et al. 2011).
8.5 Mechanisms Underlying the Capsaicin Antitumor Activity
In spite of the well-known antiproliferative effect of capsaicin in tumor cell lines, the mechanism underlying the intracellular signaling pathways are poorly understood. In this section, we will summarize the best established intracellular phenomena as well as recent pathways involving AMP-dependent kinase (AMPK) and autophagy.
8.5.1 Intracellular Calcium
The calcium dependence of apoptosis has been well defined and comprises a sustained elevation of intracellular calcium as well as a decrease in endoplasmic reticulum calcium. Sustained elevation in intracellular calcium concentration activates various secondary mechanisms that induce the increased production of reactive oxygen and nitrogen species and ultimately the programmed cell death. Several studies showed that capsaicin-induced apoptosis via increased Ca2+ levels in different carcinoma cells models (Chou et al. 2009; Huang et al. 2009; Lu et al. 2010), although the molecular mechanisms of the Ca2+ signaling pathways leading to capsaicin-induced cell death are still misunderstood. The release of intracellular Ca2+ may be an important regulatory factor on early apoptosis induced by capsaicin as calcium chelators prevent capsaicin-induced cell death (Lee et al. 2009). In most cancer cell types other than neuroblastoma or glioma cells, the increase in intracellular calcium is secondary to ER stress-dependent responses rather than a massive calcium entry by a calcium-permeable channel (Huang et al. 2009; Lee et al. 2009; Ip et al. 2012).
8.5.2 Reactive Oxygen Species
Reactive oxygen species (ROS), including superoxide (O2 −), hydroxyl radical (HO−), and hydrogen peroxide (H2O2), have a dual role in cellular environments. Whereas low ROS levels are involved in normal cell events, excess ROS cause cellular damage and ultimately lead to cell death (Sinha et al. 2013). The involvement of ROS in vanilloid-induced apoptosis has been demonstrated in most of the cell lines analyzed (Sanchez et al. 2007; Huang et al. 2009; Lu et al. 2010) and revised by Surh (Surh 2002; Lu et al. 2010). In recent years, a number of studies have shown that oxidative stress could cause cellular apoptosis via both the mitochondria dependent and mitochondria-independent pathways (Sinha et al. 2013).
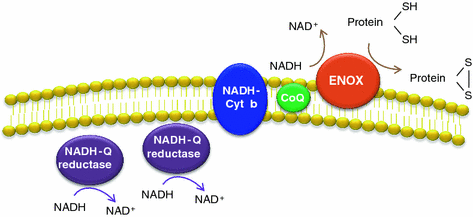
The plasma membrane of cells contains an electron transport chain (tPMET), cyanide-insensitive, comprised by two cytoplasmic NADH-coenzyme Q reductases, one NADH cytochrome b reductase, the lipophilic electron carrier coenzyme Q (CoQ), and a terminal coenzyme Q oxidase located on the external plasma membrane surface (Fig. 8.3). The last one oxidizes externally supplied NADH and hence they are NADH oxidases (NOX). Since they are located on the outside cell surface, they are referred to as ECTO-NOX or ENOX proteins (Crane and Low 2008; Jiang et al. 2008). The tPMET reduces extracellular oxidants by using the reducing power of intracellular antioxidants, making the cell metabolism respond to changes in the local redox environment. They also exhibit protein disulfide-thiol interchange activities (Bosneaga et al. 2008). In tumor cells, this plasma membrane NADH oxidase is upregulated (Morre et al. 1995). It seems that at least one function of this redox system is to regenerate NADP from NADH accumulated in the glycolytic production of ATP, increased in tumor cells. CoQ blockage inhibits the plasma membrane NADH-oxidoreductase electron transport chain and promotes reactive oxygen species production that may result in apoptosis.