Sandra P. Welch, PhD, and Robert Malcolm, MD
13
SUBSTANCES INCLUDED
Δ9-Tetrahydrocannabinol (THC), the major psychoactive ingredient in marijuana, first was isolated and purified in 1965. More than 400 chemicals are synthesized by the hemp plant, approximately 60 of which are cannabinoids. Endogenous ligands, which bind to cannabinoid receptors, include arachidonoylethanolamide (anandamide), 2-arachidonoylglycerol (2-AG), noladin ether, virodhamine, and N-arachidonoyldopamine. These lipid signaling molecules are referred to as endocannabinoids. There are two known cannabinoid receptor subtypes, CB1 and CB2. The discovery of the receptors led to the development of numerous receptor agonists and antagonists. These include the CB1 cannabinoid antagonist/inverse agonist, SR141716A (rimonabant), and the cannabinoid CB2 receptor antagonist, SR144528. Sativex (a 1:1 mixture of THC and cannabidiol) has been approved to treat spasticity from multiple sclerosis. A mixed ratio of THC and cannabidiol in capsular formulation is also available. In addition, synthetic THC (dronabinol, Marinol) is available, as is nabilone (Cesamet), a synthetic cannabinoid with therapeutic use as an antiemetic, as an appetite stimulant, and as an adjunct analgesic for neuropathic pain.
FORMULATIONS AND METHODS OF USE AND ABUSE OF NATURAL CANNABINOIDS
The concentration of THC varies among the three most common forms of cannabis: marijuana, hashish, and hash oil. Marijuana is prepared from the dried flowering tops and leaves of the harvested plant. Hashish, dried cannabis resin and compressed flowers, has 2% to 8% THC content. Hash oil, obtained by extracting THC from hashish or marijuana with an organic solvent, is a highly potent substance with between 15% and 50% THC concentration. The “fiber-type” cannabis has low THC content (typically <0.4%) coupled with high cannabidiol content.
SYNTHETIC CANNABINOIDS: “SPICE”
Synthetic cannabinoid-like compounds represent a diverse group of pharmacologic agents that are agonists or partial agonists at endogenous CB1 receptors. They are sold under names such as Spice, K2, and many rapidly changing names. A number of these compounds have not been characterized or scheduled as control substances by the Drug Enforcement Administration. They are not detected in standard urine drug screens.
CLINICAL USES
Antiemetic Effect
Two oral formulations described previously, dronabinol (Marinol) and nabilone (Cesamet), are approved by the U.S. Food and Drug Administration (FDA) to treat emesis refractory to conventional antiemetics, as well as related cachexia. Side effects such as dizziness and dysphoria limit the use of such drugs.
Appetite Stimulation and Cachexia
The appetite-stimulating effects of THC (mediated by CB1 receptor activation) led to development of the CB1 receptor antagonist SR141716A (rimonabant) as an agent for weight reduction. In June 2007, the FDA’s Endocrine and Metabolic Drugs Advisory Committee voted against recommending rimonabant for approval, due to enhancement of mood disorders and depression. Rimonabant was withdrawn from the market in late 2008.
Anticonvulsant Effect
The role of the endocannabinoid system in the regulation of neuronal firing, action potential modulation, and excitotoxicity has led to numerous studies of the role of CB1 receptors in epileptiform activity. These findings indicate that the CB1 receptor could be a therapeutic target for the treatment of epilepsy and other diseases resulting in seizures.
Neurologic and Movement Disorders
There are numerous anecdotal reports that smoked cannabis relieves spasticity arising from multiple sclerosis and spinal cord injury. The endocannabinoid system is subject to plasticity changes of various durations in pathologic conditions such as neurologic, neuropsychiatric, and movement disorders.
Analgesia
CB1 receptors are expressed at high levels in a variety of peripheral and central neurons that participate in pain perception. CB1 agonists produce analgesia by acting at several sites along pathways for pain transmission peripherally, spinally, and supraspinally. The endocannabinoids are targets of the majority of studies of CB1 and CB2 receptors in the modulation of pain. In addition, modulators of the synthesis, transport, and degradation of the endocannabinoids have become increasingly important therapeutic targets. Anandamide (AEA) and 2-arachidonoylglycerol (2-AG) produce antinociception when administered to animals. The identification of fatty acid amide hydrolase (FAAH) as the enzyme primarily responsible for anandamide catabolism and the serine lipase monoacylglycerol lipase (MGL) responsible for 2-AG degradation has provided valuable targets to increase endogenous levels of each of these respective endocannabinoids. Thus, FAAH inhibitors such as URB-597 as well as MGL inhibitors produce antinociception. There is increasing evidence that the CB2 receptor is a critical component of inflammatory pain, in addition to having multiple effects on inflammation, autoimmune responses, and bone density, all potential players in the etiology of such pain.
Glaucoma
The synthetic cannabinoid nabilone is marketed in Europe for the treatment of glaucoma. Recent reviews also include the potential for the endocannabinoid tone to play a role of potential therapeutic use in the treatment of glaucoma.
NONMEDICAL USE, ABUSE, AND DEPENDENCE
Dependence
Numerous studies demonstrate tolerance and dependence to THC in animals and a wide variety of behavioral test systems. Both the DSM-IV-TR and the World Health Organization’s International Classification of Diseases recognize cannabis dependence.
Withdrawal
A cannabis abstinence syndrome is observed in human experimental studies and includes effects that are typically the opposite of those produced by the drug, such as insomnia, anorexia, anxiety, irritability, depression, and tremor. The characteristics of cannabis withdrawal are those of a true drug withdrawal syndrome, although the predominant symptoms are behavioral and affective, rather than physical. The amount of cannabis consumed and the duration of use are critical components of the intensity and duration of the withdrawal syndrome.
HISTORICAL FEATURES
The use of cannabis dates back over 12,000 years. Cannabis use is believed to have started in central Asia and continued to flourish in Southeast Asia and India. It is believed that cannabis was introduced into the Americas in the 1600s by the English settlers and Spanish conquistadors. The earliest references to its medicinal uses date back to 2700 bc and included treatment for constipation, malaria, rheumatic pains, and female disorders. The euphoric properties were discovered in India around 2000 bc, and cannabis was recommended for reducing fevers, producing sleep, stimulating the appetite, relieving headaches, and curing venereal diseases.
EPIDEMIOLOGY
Worldwide, cannabis is the most widely used illicit substance. There was a slight increase in the worldwide prevalence of marijuana use from 2009 to 2012 with mean age of use about 17.5 years, older than the mean age of 17.0 years in 2002. In 2010, ED visits for marijuana-related clinical problems increased by 64%. The 2012 Monitoring the Future survey found that 6.5% of over 45,000 8th, 10th, and 12th grade public and private school students smoked marijuana daily, up from 5.1% the prior year. About 23% of 12th graders smoked marijuana in the prior month; 36% smoked marijuana in the prior year.
NEUROBIOLOGY
Cannabinoid Receptors
The cannabinoid CB1 receptor, a saturable binding site for which cannabinoids possess high affinity, has been identified primarily in tissues of central nervous system (CNS) origin. An antagonist for the CB1 receptor, SR141716A (rimonabant), selectively attenuates cannabinoid CB1 receptor–mediated activity in vivo and in vitro. The CB2 receptor was first identified on splenic macrophages. It is found in both peripheral and central (brain) sites. A specific antagonist for the CB2 receptor is SR144528. The CB1 and CB2 receptors share 40% homology; Δ9-THC has similar binding affinity for both receptor subtypes.
Endocannabinoids
The first endocannabinoid discovered, anandamide (AEA), is one of a family of arachidonic acid derivatives that have cannabinoid effects. Another major endocannabinoid is 2-arachidonoylglycerol (2-AG), discovered in canine gut. 2-AG levels are higher in the brain than are those of AEA. Several synthetic pathways for AEA and 2-AG are now worked out. A specific phospholipase D (NAPE-PLD) has been proposed to hydrolyze N-acyl-phosphatidylethanolamine to AEA. In a similar manner, arachidonic acid is also released from membrane lipids and is subsequently converted to anandamide or 2-AG, which is metabolized by two sn-1–specific diacylglycerol (DAG) lipases (DAGLa and DAGLb) that have been identified. Degradative enzymes include FAAH as the enzyme primarily responsible for AEA catabolism (Fig. 13-1) and the serine lipase MGL (Fig. 13-2) responsible for 2-AG degradation. AEA and 2-AG are synthesized “on demand” and then released following cell depolarization or the mobilization of intracellular calcium stores. The process requires activation of Gq/G11 protein–coupled receptors.
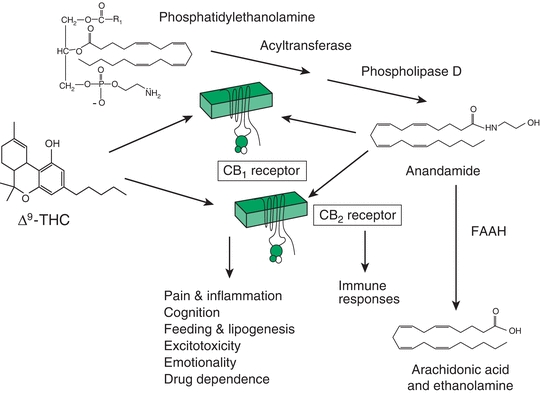
FIGURE 13-1. Endocannabinoid system.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


