(1)
University of South Australia, Adelaide, Australia
Abstract
This chapter presents “The pharmaceutical R&D financing” game in which the Institution lowers the decision threshold and must respond to a pharmaceutical industry Threat. Firms claim that the capital market fails to provide funds for pharmaceutical Research and Development (R&D); it is a risky investment and returns are long-term. Hence, firms finance R&D through internal funds generated by above marginal cost pricing of drugs. If institutions use monopsonist power to bargain down prices, there will be insufficient funds to finance the R&D that society requires. The population will be worse off. This claim leads to the following paradox: Why should the health budget finance pharmaceutical R&D without a formal contractual arrangement if firms and pharma-economists are claiming that the capital markets are unwilling to take on this risk, even with the protection of legally enforceable contracts? The Game is structured as a choice by firms between the two strategies to raise funds: Lobby (approach the Institution to increase prices) or Borrow (go to the capital market). I conclude that there is no incentive for the Institution to finance pharmaceutical R&D through higher prices, unless a contract is negotiated. However, in this case, if institutions are more risk adverse than banks, this strategy (Lobby with Contract) will be more expensive for firms than approaching the capital market. The practice by firms of financing most of their investment in R&D from internal funds is more likely to be the result of these internal funds being imperfectly priced by institutions, not failure in the capital market.
9.1 The Reimburser’s Problem
A Firm approaches the Reimburser with a new drug and evidence of its additional cost, ΔC P , and effect, 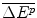 . This new drug is substantially more effective than the best available existing therapy
. This new drug is substantially more effective than the best available existing therapy 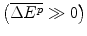 . The Firm’s offer IPER, f, is much higher than the health shadow price (f ≫ β c ). These two conditions mean that the drug’s reimbursement will have a significant budgetary impact, in this case:
. The Firm’s offer IPER, f, is much higher than the health shadow price (f ≫ β c ). These two conditions mean that the drug’s reimbursement will have a significant budgetary impact, in this case:
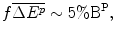
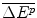 . This new drug is substantially more effective than the best available existing therapy
. This new drug is substantially more effective than the best available existing therapy 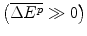 . The Firm’s offer IPER, f, is much higher than the health shadow price (f ≫ β c ). These two conditions mean that the drug’s reimbursement will have a significant budgetary impact, in this case:
. The Firm’s offer IPER, f, is much higher than the health shadow price (f ≫ β c ). These two conditions mean that the drug’s reimbursement will have a significant budgetary impact, in this case: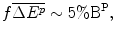
where B P is the drug budget. Existing non-drug programmes with an aICER of d will need to be displaced to provide the funds from a fixed health budget to finance the new drug. These programmes are unpatented, non-pharmaceutical programmes such as respite care and free dental services. The budget is currently economically efficient (n = m) and displacement is optimal (d = m) therefore:
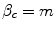
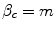
The Firm argues that an IPER of f, where f > β c , will ensure that there are sufficient internal funds to finance the development of a future drug. The Firm claims that it will invest the entire premium over β c into new drug R&D. This investment is an amount 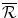 where:
where:
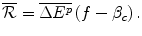
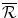 where:
where: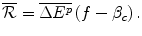
The Firm argues that it needs to fund R&D from internal funds because the capital market fails to finance pharmaceutical R&D; even with formal contracts, the investment is too risky and the returns, if they occur, are too long-term. The Firm supports this argument with peer reviewed papers and US government reports that state that capital market imperfection limits pharmaceutical firms’ access to external funds and hence firms rely on internal funds generated from additional profit from higher prices (Vernon 2003; International Trade Administration 2004; Santerre and Vernon 2006). These authors conclude that without funds from economic rent there will not be enough funds available to finance the R&D that society requires. Therefore, lower prices, which will reduce the amount of economic rent and hence internal funds, are not in the long-term interest of consumers. The Firm’s case for financing pharmaceutical R&D using funds sourced from higher prices appears strong.
The Firm also provides the Reimburser with a US Congressional Budget Office Report (2006) that refers to Reinhardt (2001)1 in which the Report’s authors write that:
A relatively close relationship exists between drug firms’ current R&D spending and current sales revenue for two reasons. First, successful new drugs generate large cash flows that can be invested in R&D (their manufacturing costs are usually very low relative to their price). Second, alternative sources of investment capital—from the bond and stock markets—are not perfect substitutes for cash flow financing. Those alternative sources of capital are more expensive because lenders and prospective new shareholders require compensation (in the form of higher returns) for the additional risk they bear compared with the firm, which has more information about the drug under development, its current status, and its ultimate chance of success. (p. 9)
The Firm uses this statement to lend support to their argument that if the Firm is required to go to the Capital Market to finance its R&D, it will be more costly and the price of future new drugs will need to be increased to compensate for this.
The Reimburser is confused. What does it mean to say that the Firm “has more information about the drug under development, its current status, and its ultimate chance of success”? What sort of information do companies have prior to conducting an RCT? What information do they have that is not understood by the Capital Market? Can this information be provided to and understood by Institutions? The Reimburser is reminded of a paper she read recently on the benefits of risk-sharing arrangements for pharmaceuticals and the economics of warranties where the authors describe warranties as a method of signalling high quality when it is not observable and the costs of measuring that quality are high (Cook et al. 2008, p. 555).
The Reimburse asks: “What are these unobservable aspects of quality? What is this information firms have and why would they not put it in the public domain? How are they going to obtain this information without costly RCTs?”
The US literature does provide alternative explanations for why large pharmaceutical firms use internal funds. Hall (2002) identified a number of possible reasons, including information asymmetry, moral hazard on the part of firms and a preference by the capital market for collateral in the form of physical assets. She concludes that there is a possible case for government subsidies for smaller (start-up) firms. Hall also concludes that it is hard to establish any evidence of a “financing gap” for large established firms, but that it is possible to identify a preference for internal funding (Hall 2002 p. 49).
The Reimburser recognises that there could be an alternative explanation for the observation that firms finance R&D from internal funds. She hypothesises that firms prefer to finance through internal funds (financed by economic rent) because this method is cheaper than raising funds through the capital market. It is cheaper because, unlike the capital market, the Reimburser does not require firms to present a case for financing the NME’s R&D, nor does it require the firm to agree to a contract that sets out the capital repayments and interest payments.2
Financing pharmaceutical R&D from the health budget comes at a tangible and significant cost to the population’s health today. The Reimburser reviews the proposed budget cuts for programmes over the next 3 financial years as the whole of government responds to the increasing pressure of government debt. In this climate, services that are no less cost-effective that the new drug will need to be displaced to finance the pharmaceutical R&D premium. This displacement occurs in addition to the displacement to finance the additional costs of the health effects from the new drug. Furthermore, there is no contractual arrangement with the Firm to guarantee a return to consumers on their risky investment: their own health today foregone to increase the population’s health tomorrow.
The Reimburser wonders how risky it is to use the health budget to finance pharmaceutical R&D and whether her population can bear this risk. After all, firms claim that the capital market finds it too risky to lend to firms, and unlike the Reimburser, the capital market is protected by a contract.
The Reimburser asks:
How should the rational and risk-averse or risk-neutral Institution respond to the Threat?
9.2 The Pharmaceutical R&D Financing Game
We use a game theoretic model to identify the underlying strategies and payoffs in the Pharmaceutical R&D Financing Game. The Game is then used to make a number of predictions that are tested against real world observations and identify the conditions required for the risk-averse Institution to respond to this threat by increasing the threshold price above β c . Finally we consider whether, if these conditions are met, the Firm will continue to have a preference for funding R&D from internal funds rather than the capital market.
An adaption of Grüne-Yanoff and Schweinzer’s Architecture of Game Theory (Grüne-Yanoff and Schweinzer 2008) is used to develop the story as an applied game. This framework comprises the following elements (1) World (the economic problem); (2) Game, which comprises the narrative and the game structure; and (3) Theory Proper. This framework is detailed in Chaps. 2 and 6 and Pekarsky (2012, Appendix 1). The following three Sects. (9.3, 9.4 and 9.5) set out these components, and then the solution is presented in Sect. 9.6.
9.3 World (The Economic Problem)
Firms use internal funds rather than the capital market to finance pharmaceutical R&D. This is because internal funds are less costly than capital market funds due to imperfections in the capital market, identified by authors such as Santerre and Vernon (2006). These imperfections are a consequence of the capital market being unable to incorporate the full long-term benefits to society of investments in pharmaceutical R&D. They are also a consequence of information asymmetry; according to a US Congressional Report (2006), firms have information about the future benefits of a drug that is not available to capital markets or is costly to make available. Firms use the evidence of funding preferences and the associated economic rationale sourced from the peer reviewed literature to provide an evidence base for the following threat:
The FPP is the price that is necessary in order to ensure that sufficient R&D is available for the future. If prices are lower, then funds will need to be sourced from the capital market rather than internal funds. This will increase the costs of capital and combined with the lower prices, will mean that firms will reduce investments in R&D and hence there will be fewer new drugs in the future and the population will be worse off.
How should the rational Institution respond to this threat?
9.4 Model
The Model comprises the Narrative and the Game Structure.
9.4.1 Narrative
The Game starts when the Firm decides whether to raise the funds for R&D from either the health budget (Lobby) or capital market (Borrow). The Firm also has the option to do Nothing.
9.4.1.1 Lobby
The Firm’s first option is Lobby (L): lobby purchasers to pay higher prices for the existing drug. This lobbying process uses various submissions, reports and delegations to influence decisions that impact the price of a new drug and hence the profit for a given quantity of the new drug sold. The Lobbyists’ key claim is that purchasers must not use their monopsony powers to negotiate prices below the offer price because if this occurs there will be insufficient funds available to finance the R&D required for future drugs. The Lobbyists’ position can be summarised as: only economic rent can finance R&D efficiently, and reduced economic rent means proportionally less R&D and less R&D means fewer new drugs and hence less health in the future.
The Institution can either reject or accept the Firm’s lobbying. If it rejects the lobbying, the game ends. If the lobbying is successful, a higher price for the existing drug is agreed upon and the additional economic profit that results from this lobbying provides additional internal funds that are invested in NME R&D. These additional funds are sourced by the higher prices on existing drugs, which are in turn financed from the fixed health budget by displacing existing services that have an aICER of d. These services are the least cost-effective of existing services (displacement is optimal). The budget is currently economically efficient; there are no alternative ways of producing health within existing technologies and resources that will improve health for the population.3 Therefore, because the conditions of optimality of displacement, d = m, and economic efficiency, m = n, are met and the budget is fixed, we conclude that β c = m.
The Firm’s investment in pharmaceutical R&D might or might not result in an NME; there is a risk. This risk is characterised inconsistently in the literature.4 However, the claim by the Lobbyists is that this risk is so high and the return, if it occurs, is so far into the future that the capital market will not finance this R&D, or alternatively, will finance this R&D at prohibitively high rates. In the context of this model, this risk is simply expressed as a probability, q, that an NME of a given value of clinical innovation will be brought to the market and a probability (1 − q) that there will be no NME brought to the market as a consequence of the Firm’s decision to invest in new drug R&D.
If the R&D is not successful, the Game ends and there are no mechanisms in place for the Institution’s investment to be returned, so there is a loss to the Institution equivalent to its original investment. If the R&D is successful then the Firm selects an offer price for the future drug and the Institution can choose to reimburse the future drug at the offer price or do nothing. (The details of this part of this Game are addressed in Game 1 Sect. 8.6.1.1.)
9.4.1.2 Borrow
The second R&D financing option available to the Firm is Borrow (B): go to the Capital Market and attempt to borrow all the funds required for the development of an NME. The process of borrowing to finance the costs of a specific project requires the Firm to present a Bank with evidence of its financial status, the funds it requires, and the likely success of its investment in terms of future revenue and profit. This estimate of future profit would need to include an assumption about the future revenue from the future drug, which would in turn require assumptions about: the potential clinical innovation of the future drug, 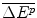 ; the estimated costs of producing the additional health effects (the IMER, in this case c per QALY); the market share of the future drug; the future threshold price; and the IPER of the future drug.
; the estimated costs of producing the additional health effects (the IMER, in this case c per QALY); the market share of the future drug; the future threshold price; and the IPER of the future drug.
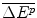 ; the estimated costs of producing the additional health effects (the IMER, in this case c per QALY); the market share of the future drug; the future threshold price; and the IPER of the future drug.
; the estimated costs of producing the additional health effects (the IMER, in this case c per QALY); the market share of the future drug; the future threshold price; and the IPER of the future drug.We assume that if the R&D is unsuccessful then there is no repayment of the loan. This assumption is a simplification but it is consistent with the observation that there is limited physical collateral held by the pharmaceutical Firm and that this is a factor influencing the decision by the Capital Market to lend to Firms. It also allows the riskiness of the loan to be characterised as part of the payoff to the Capital Market.5
The Bank (a lender in the Capital Market) will review the case presented by the Firm and solve for the Bank’s minimum acceptable interest rate. The Bank’s choice of offer price (interest rate), θ, will also be influenced by its assessment of the Firm’s maximum acceptable price. We assume the Bank selects an offer price of θ. The Firm can either reject or accept the Bank’s proposal to lend at a rate θ, or it may enter into a negotiation if its maximum acceptable interest rate is higher than the Bank’s minimum acceptable rate. If the Firm and the Bank agree on an interest rate, the Firm will Borrow an amount, ℛ, with a requirement that it pays an interest rate of θ, as well as repaying the loan from the revenue from the future drug, should the R&D be successful. A contractual arrangement ensures repayment if there is success and sets out the shared understanding of the risks associated with the loan. If there is no success, there will be no repayment. This condition, which is set out in the contract, makes the loan “high risk”. It characterises the claimed failure of the Capital Market to finance this R&D due to high risk.
The Bank has a second option; lend to a risk-free borrower at a rate of τ, therefore its payoff from lending to the Firm is net of the opportunity cost of this foregone activity. This use of an economic payoff for the Bank is consistent with the use of an economic payoff for both the Firm and the Institution.
If the R&D is successful, the Firm will offer the NME to the Institution at an IPER = f. The Institution will either reimburse the future drug at this price or do nothing. If it rejects the drug at this price, then the Game ends. There will be no repayment to the Bank because there is no revenue stream associated with the future drug, despite the success of the R&D in bringing a drug to market.
9.4.1.3 Some Other Parts to the Story
We assume that the expected incremental effect of the drug, 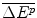 , is independent of the method used to finance the future drug. The probability of success (q) or failure (1 − q) of the R&D process is also assumed to be independent of the method used to finance the R&D for the future drug. While the Firm would need to present a business case to the Bank to support its application for a loan, it is assumed that no such documentation is required if the Firm chooses to lobby the Institution to obtain these funds. And while the Firm is required to repay the loan and pay interest to the Bank if there is success, it is not required to make such payments to an Institution if the R&D is successful.
, is independent of the method used to finance the future drug. The probability of success (q) or failure (1 − q) of the R&D process is also assumed to be independent of the method used to finance the R&D for the future drug. While the Firm would need to present a business case to the Bank to support its application for a loan, it is assumed that no such documentation is required if the Firm chooses to lobby the Institution to obtain these funds. And while the Firm is required to repay the loan and pay interest to the Bank if there is success, it is not required to make such payments to an Institution if the R&D is successful.
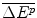 , is independent of the method used to finance the future drug. The probability of success (q) or failure (1 − q) of the R&D process is also assumed to be independent of the method used to finance the R&D for the future drug. While the Firm would need to present a business case to the Bank to support its application for a loan, it is assumed that no such documentation is required if the Firm chooses to lobby the Institution to obtain these funds. And while the Firm is required to repay the loan and pay interest to the Bank if there is success, it is not required to make such payments to an Institution if the R&D is successful.
, is independent of the method used to finance the future drug. The probability of success (q) or failure (1 − q) of the R&D process is also assumed to be independent of the method used to finance the R&D for the future drug. While the Firm would need to present a business case to the Bank to support its application for a loan, it is assumed that no such documentation is required if the Firm chooses to lobby the Institution to obtain these funds. And while the Firm is required to repay the loan and pay interest to the Bank if there is success, it is not required to make such payments to an Institution if the R&D is successful.The relationships between the additional cost, additional effect and IPER compared with the best existing drug are detailed in Game 1, Sect. 8.6.1.1. For example, the IPER of the new drug is assumed to be the result of a higher price for the new drug, and no additional savings elsewhere in the health budget are expected.
9.4.1.4 The Rules of Engagement
1.
If the Institution is indifferent between reimbursing that drug at the offer price and the best alternative action then the Institution must accept the Firm’s offer price for the future drug.
2.
The Institution cannot negotiate below the offer price if the offer price is at or below the decision threshold.
9.4.1.5 The Threat
The FPP is the price that is necessary in order to ensure that sufficient R&D is available for the future. If prices are lower, then capital funds will need to be sourced from the capital market rather than internal funds. This situation will increase the costs of capital and combined with the lower prices, firms will reduce investments in R&D and hence there will be fewer new drugs in the future and the population will be worse off.
9.4.2 The Game Structure
Game 2 is presented in extensive form as a dynamic game (there is a sequence of decisions) of incomplete information (there is uncertainty in the payoff to R&D, but not in the probabilities of success and fail) and no private information (all information that is certain is in the public domain, including the value of the probability of success or ail of R&D). Even though the process of raising R&D funds, developing new drugs and obtaining reimbursement occurs over a period of several years, the Game is represented as occurring in one period. This simpler specification allows the key strategic incentives to be identified. In Chap. 10 the findings from Games 1 and 2 inform a three-period game of the drug R&D process; Game 3.
9.4.2.1 Extensive Form Representation of the Game
The extensive form representation of the Game is presented in Fig. 9.1.
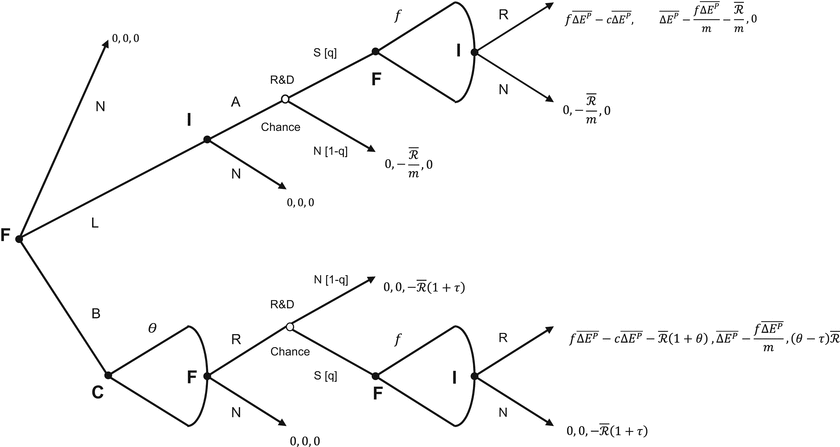

Fig. 9.1
The pharmaceutical R&D financing game
9.4.2.2 Players, Actions and Payoffs
There are three players: the Firm (F), the Institution (I) and the Capital Market (C). The payoffs in the Game are listed in that order in Fig. 9.1.
Game 2 starts when the Firm approaches either the Capital Market or the Institution to raise the funds required to develop an NME. The Game in Fig. 9.1 sets out three actions available to the Firm in the first stage: do Nothing (N), Lobby (L) or Borrow (B). The details associated with each action follow.
Firm Chooses to Do Nothing (N)
If the Firm chooses to do Nothing, the Game ends and the payoff to each player is zero. The players will all continue to make profits (F and C) or health gains for the population (I) from existing activities, the outcomes of which are assumed not to be impacted by this particular Game.
Firm Chooses to Lobby (L)
Lobbying (L) involves the Firm making a case to the Institution that it should provide the research funds  via higher prices on the existing drug. If the Firm chooses to Lobby (L) the Institution, the Institution can choose to either Accept (A) or do Nothing (N) in response to the proposal by the Firm to raise additional funds through internal revenue (economic rent) on the existing drug. If the Institution chooses to do Nothing (N) in response to the Lobbying (L), the Game ends and the payoff to each of the Firm, the Institution and the Capital Market is zero.
via higher prices on the existing drug. If the Firm chooses to Lobby (L) the Institution, the Institution can choose to either Accept (A) or do Nothing (N) in response to the proposal by the Firm to raise additional funds through internal revenue (economic rent) on the existing drug. If the Institution chooses to do Nothing (N) in response to the Lobbying (L), the Game ends and the payoff to each of the Firm, the Institution and the Capital Market is zero.
 via higher prices on the existing drug. If the Firm chooses to Lobby (L) the Institution, the Institution can choose to either Accept (A) or do Nothing (N) in response to the proposal by the Firm to raise additional funds through internal revenue (economic rent) on the existing drug. If the Institution chooses to do Nothing (N) in response to the Lobbying (L), the Game ends and the payoff to each of the Firm, the Institution and the Capital Market is zero.
via higher prices on the existing drug. If the Firm chooses to Lobby (L) the Institution, the Institution can choose to either Accept (A) or do Nothing (N) in response to the proposal by the Firm to raise additional funds through internal revenue (economic rent) on the existing drug. If the Institution chooses to do Nothing (N) in response to the Lobbying (L), the Game ends and the payoff to each of the Firm, the Institution and the Capital Market is zero.If the Institution chooses to Accept (A) the Lobbying (L) and pay the Firm the additional economic rent, 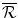 per unit sold, the Firm will invest the entire funds6 into the R&D and the result will be either Success (S), an NME, or No success (N), no new drug. The probability of these two outcomes is q and 1 − q respectively. If there is No success (N) the game stops and the payoffs to the Firm and the Capital Market are both zero, whereas the financial payoff to the Institution is the net financial cost for the population’s health budget:
per unit sold, the Firm will invest the entire funds6 into the R&D and the result will be either Success (S), an NME, or No success (N), no new drug. The probability of these two outcomes is q and 1 − q respectively. If there is No success (N) the game stops and the payoffs to the Firm and the Capital Market are both zero, whereas the financial payoff to the Institution is the net financial cost for the population’s health budget: 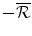
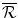 per unit sold, the Firm will invest the entire funds6 into the R&D and the result will be either Success (S), an NME, or No success (N), no new drug. The probability of these two outcomes is q and 1 − q respectively. If there is No success (N) the game stops and the payoffs to the Firm and the Capital Market are both zero, whereas the financial payoff to the Institution is the net financial cost for the population’s health budget:
per unit sold, the Firm will invest the entire funds6 into the R&D and the result will be either Success (S), an NME, or No success (N), no new drug. The probability of these two outcomes is q and 1 − q respectively. If there is No success (N) the game stops and the payoffs to the Firm and the Capital Market are both zero, whereas the financial payoff to the Institution is the net financial cost for the population’s health budget: 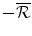
The payoff to the Institution is the loss of the Institution’s investment of health budget funds into the R&D process raised via the increased price of existing drugs paid to the Firm as a consequence of lobbying. The health payoff to the Institution if there is No success (N) is the net change in the population’s health:
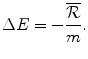
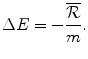
The payoff is the loss in health effects as a consequence of financing the pharmaceutical R&D by displacing services with an aICER of m. The health budget is currently economically efficient and displacement is optimal (d = m) therefore the NEBh (which is negative) is the same as the net health loss for the population.
If the R&D is successful, then the Firm will offer the future NME at an IPER of f. The Institution will either Reimburse (R) or do Nothing (N) when the Firm presents the future drug at the offer price of f. If the Institution Reimburses (R) the drug at the offer price, the payoff to the Firm, π F is:
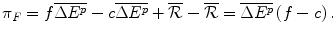
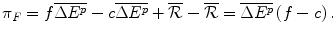
This payoff comprises:
The revenue from the new drug that has an IPER f where the total additional health effects for the target patients are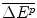 and hence revenue is
and hence revenue is 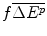 ;
;
Less the costs of production of the future drug, an IMER of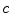 per unit additional health effect produced (
per unit additional health effect produced (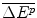 ) and hence a total manufacturing cost of
) and hence a total manufacturing cost of 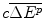 ;
;
Plus the amount raised by lobbying,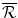 ; and
; and
Less the investment into NME R&D,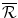 .
.
The Lobbying is assumed to be costless; an assumption that is relaxed in the discussion.
The payoff to the Capital Market will be zero and the payoff to the Institution, the net health benefit to the population, ΔE will be:
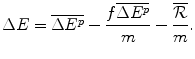
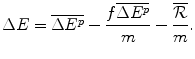
This payoff comprises:
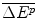
The additional health effects of the future new drug for target patients, compared with the best existing therapy in the future;
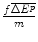
The health effects lost due to the requirement to displace an amount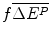 of services with aICER = m to finance the future drug; and
of services with aICER = m to finance the future drug; and
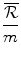
The health effects displaced to finance the additional amount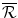 .
.
If the Institution chooses to Not reimburse (N) the future NME at the offer price, the payoff to the Firm and the Bank is zero and the payoff to the Institution is the incremental change in the population’s health: