, Mohammed Al-Rubaie2, Stuart Walker3 and Sam Salek4
(1)
Regulatory Affairs Consultant Executive Directive, Kuwait Advancement for Conference and Exhibition Management, Kuwait, Kuwait
(2)
Ministry of Health, Directorate General of Pharmaceutical Affairs and Drug Control, Muscat, Oman
(3)
Founder of Centre for Innovation in Regulatory Science, London, UK
(4)
Department of Pharmacy School Life & Medical Sciences, University of Hertfordshire, Hatfield, UK
Introduction
The primary aim of drug regulation is the protection of public health. However, Hill and Johnson (2004) suggested that for some the balance between regulating pharmaceuticals in the interests of ensuring public health and encouraging the development of pharmaceutical products has shifted in favour of the innovative industry. Furthermore, regulation of pharmaceuticals has been perceived as an obstacle to the availability of medicines in national or regional markets, and this has placed a significant demand on regulators to expedite reviews and evaluations to approve medicines (Moore and Ferburg 2014). Nevertheless, medicines regulation is the foundation of any country’s national drug policy that ensures a viable pharmaceutical industry as well as a high-standard drug approval process.
The establishment of the International Conference on Harmonisation (ICH) between the United States, Europe and Japan in 1990 reflected a need felt by the research-based industry and certain governments to streamline the approval process for the registration of new medicines (Mallia-Milanes 2010). The term harmonisation refers to the standardisation of technical requirements for medicines regulation, of which the requirements relate to quality, safety and efficacy of medicines, and this can differ from one country to the other. Since its inception, ICH has evolved, through its Global Cooperation Group (GCG), to respond to the increasingly global phase of drug development. The GCG was originally formed as a subcommittee in 1999, in response to the growing interest in ICH guidelines beyond the ICH regions, and one such harmonisation was that of GCC, the Gulf Cooperation Council. This collaborative mechanism ensures a more transparent and streamlined process for the marketing authorisation of pharmaceutical products in the GCC region.
The Gulf centralised registration process is facing several challenges which has led to delays in patients’ access to medicines with the need to re-register products in individual countries after central registration. In addition there is a requirement to register the manufacturer prior to submitting the product file to the Central Registration Office. Currently, there is a need to establish effective methods for sharing the workload amongst the regulatory authorities in the GCC states. A paucity of studies at the GCC level, probing into the views and experiences of individual pharmaceutical companies with GCC central registration system, has made its improvement a constant challenge. Hence, it is envisaged to explore the importance of the system and identify challenges and critical success factors to enhance the effectiveness and efficiency of the central registration procedure. Patients benefit from the timely access to safe and effective therapies whilst pharmaceutical companies are able to market the product sooner and generate revenues for further research and development.
The Views of the Pharmaceutical Companies About the Centralised Procedure
The views of the pharmaceutical companies were gathered by, initially, preparing a list including all those who had interactions with the national registration system and/or the CP in the GCC region. This list of the companies registered with the CP was obtained from the GCC-DR office in Saudi Arabia. The profile of each company was analysed to ascertain their suitability and their willingness to engage with the process. Fifty generic and 50 innovative companies were selected for this exercise from the list of registered companies, half of them with experience of registering their products through the CP whilst the other half had the experience of registering their products through the national system. Of these, 30 companies agreed to engage with the exercise. The remaining, who did not wish to take part, could be categorised into four groups:
Unwilling to participate
Lack of experience
Lack of response
Sensitivity of the information required
Companies’ General Views About the Centralised Procedure
In this section, the companies’ experiences in registering their products through the Centralised Procedure were examined. Their preference for using this system, national versus centralised, was determined. Data on the number of products submitted and registered through the Centralised Procedure, along with their experience and opinion on the system itself, were also recorded. Generally, more than half of the companies who responded preferred the national registration procedure to the Centralised Procedure (Fig. 9.1).
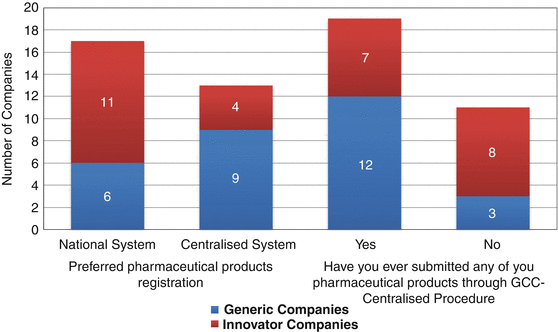

Fig. 9.1
Number of companies and their preferred pharmaceutical registration system
The majority of the pharmaceutical companies preferred to register their products through the national system than the centralised system. Eleven innovative companies preferred the national system rather than the CP whilst six generic companies choose the national system instead of the CP. Nineteen companies had submitted their products for registration under the centralised system, whilst more generic companies (12) had submitted their products to the CP than the innovative companies (Fig. 9.1).
Only the 19 companies who had the experience of registering their products through the CP were able to provide critical information on the strengths and weaknesses of the CP registration system. The main reasons for choosing the centralised registration system over the national system are illustrated in Fig. 9.2. This shows that 14 pharmaceutical companies were able to meet the requirements to GCC tender specification, convenience (12 companies) and timeliness of registration, cost/resources required and ease of subsequent CP registration (Al-Rubaie 2013). One of the companies indicated that the central registration team is more accessible, easier to communicate with, flexible and understanding about the companies’ issues. It is also faster in approval than the national authority. Another respondent indicated their choice of product registration system would be dependent on their company’s strategy and the nature of the product.
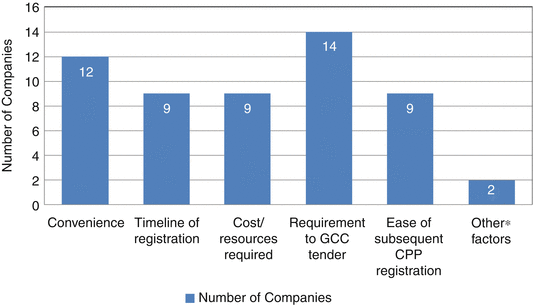

Fig. 9.2
Reasons for companies’ preference in using the Centralised Procedure for product registration (*Other factors: (1) Central registration team more accessible, easier to communicate with, flexible and understanding about the companies’ issues. Faster in approval than the national and is a respectable authority. (2) Depending on the company strategy and the nature of the product)
As for the reasons why pharmaceutical companies did not prefer the centralised system, eight of the companies stated that their product strategy was aligned to the registration in selected GCC states, hence the preference for national registration (Fig. 9.3). The timeline for registration and the experience with the national systems registrations resulted in a total of seven responses. The rest cited the cost and resource requirement and subsequent pricing of products as well as a concern over possible rejection by GCC-DR. One of the companies highlighted that for highly specialised, orphan and important products, they preferred to go via the national procedures, since they found it more transparent and easier to communicate with the regulatory authorities. The CP is a strategic option, but due to a limited number of GCC-DR annual meetings, the companies cannot take the risk for important products that are required to be available in the countries faster, since CP may take a longer time compared with national registration.
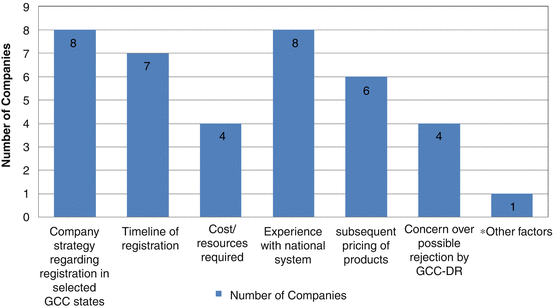

Fig. 9.3
Reasons why companies did not prefer the Centralised Procedure (*Other factors: Due to the limited number of GCC-DR annual committee meetings, the company cannot take the risk for important products registration that must be made available in the countries quickly, since it may take a long time to receive GCC-DR comments)
The number of products submitted for approval to the Centralised Procedure by 13 companies fluctuated between 28 and 75 submissions annually (Fig. 9.4).
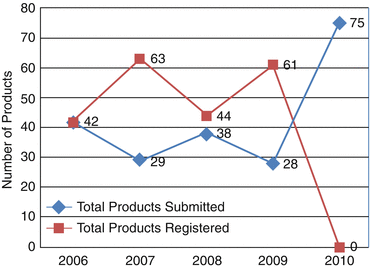

Fig. 9.4
Number of products submitted and approved through the Centralised Procedure
The year 2010 saw a substantial increase in the number of applications by almost three times as compared to the previous year (2009). This could be an indication that the companies realised the benefits of obtaining an approval from the GCC-DR to market their products to the seven member states seamlessly.
The total number of products submitted from 2006 to 2010 was 212 whilst the total products registered were 210 for the 4 years (2006–2009). An upward trend can be seen in product submissions and product registrations under the CP system. This clearly indicates that though there were challenges in processing the products for approval at GCC-DR level, it certainly is gaining recognition as a viable option. Twenty companies believed the quality of decision-making of the Centralised Procedure to be good or excellent whilst seven companies stated that it was only satisfactory. This is an indication that although there are issues with the centralised registration system, the quality of its decision-making is still satisfactory for most companies (Fig. 9.5). Although some companies had no experience of registering their products through the CP, they were still able to provide their opinion about this system.
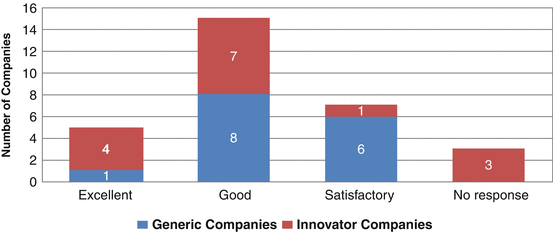

Fig. 9.5
The quality of decision-making of the Centralised Procedure
Twenty companies stated that it took them more time to register their products through the centralised system. The pharmaceutical companies also stated that the limited number of meetings by the Central Committee was one of the main causes of the delay in product registration, thus their preference for national registration. The delay in product registration is also attributed to the fact that the submission of the documents for review and approval by the Committee has to be done earlier before the summer hiatus if the companies wish to complete the procedure in good time. However, unlike the national registration procedure in which both the product and the pricing registration could be done concurrently, the company has then to wait for the product approval centrally before proceeding with the pricing approval locally.
Further delays in product approval and registration in the CP were caused by long technical document evaluation, distribution of the files for the reference countries for evaluation and analysis feedback and a long queue for submission and review by the committee. The issue of sequentially processing for national registration after the Centralised Procedure added a further delay to the approval process, and it could take between 1.5 and 2.5 years for approval to be attained for each product. Furthermore, so many issues to be discussed in the CP Committee meetings coupled with the limited number of meetings per year meant approval and registration issues would take a longer time to be resolved, hence lengthening the approval process. More delays are caused when evaluators waited until after the CP meetings to pass their comments and requests for additional information instead of asking for this immediately on reviewing at the first stage.
Twenty-seven companies indicated that the timelines for registration in the Centralised Procedure could be shortened, and 20 of these companies also stated that by having more GCC-DR meetings, this could be achieved. Others believed that having additional technical expertise and conducting parallel processing of the laboratory tests and the CP registration could also shorten the process.
Twenty of the companies also indicated that certain aspects of the registration procedure could be simplified (Fig. 9.6). The majority of these believed that electronic submissions and automation of some of the processes could assist in simplifying the Centralised Procedure as a whole and hence shorten the whole process. Others indicated that the technical evaluation could be simplified by relying on documents submitted to GCC since these were the same documents already submitted and approved in the United States/United Kingdom and other reference countries. Finally, 16 of the companies indicated that the current registration fee for the Centralised Procedure is reasonable whilst 11 of the companies stated that it is too high. Hence, the cost factor from the registration perspective is not the key reason why companies preferred the national registration system to the Centralised Procedure to obtain approval for their products.
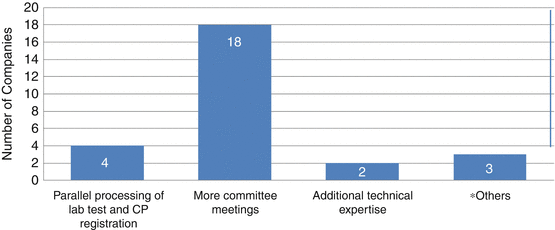

Fig. 9.6
Companies’ suggestions on how certain aspects of the registration procedure could be simplified (*Others: (1) The pricing procedure in the individual GCC states should be in parallel with the CP Registration. (2) The communication with the companies could be via email or other means of electronic transmission. (3) Stop the site inspection if the site was in a reference country and has the proper GMP and manufacturing license (same as the national registration). (4) Have one registration certificate for all GCC states from GCC-DR (once registered, no need to submit again per each country to gain approval)
Centralised Registration Procedure
The companies’ experiences in submitting and registering products under the CP are reviewed in this section. Generally the companies were satisfied with the quality of the guidelines and scientific advice as well as their opinions on the implementation of the Common Technical Documents, pharmacovigilance procedures and transparency of the Centralised Procedure. In addition, the majority of the companies also agreed that the CP led to the harmonisation of national procedures in the GCC legislation. Half of the companies indicated that the Centralised Procedure guidelines were easy to understand (eight generic and seven innovative companies), 10 companies (six innovative and four generic companies) stated that the guidelines were not always clear and another three companies indicated that they were generally unclear.
Those who stated that the guidelines were not always clear further provided their explanation for their choice. They pointed out that there were no guidelines for all registration requirements (e.g. available guidelines are for Stability Studies and Bioequivalence Study only). Variations to the guidelines were also not available. The biotechnology products registration guidelines were very short and ambiguous, and many of the requirements that the committee asked for were not included. In addition, they commented that the committee sometimes went outside the guidelines and asked for additional requirements found in other international guidelines (oscillating between EU and FDA, especially with stability and bioequivalence). They also stated that the patient information leaflet (PIL) requirements were not clear, and many times the committee asked for amendments for the PIL that was internationally recognised. Hence, they were not provided with clear criteria in this matter. Finally, they added that the registration certificates for manufacturing sites were not harmonised with regard to the nomenclature of the production lines, which created confusion when required to register a product from such a site later.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


