Fig. 20.1
Blood levels of a child exposed to doses of chloramphenicol calculated according to body weight (solid line) and blood levels in the child exposed to doses adjusted downwards to avoid the grey baby syndrome (dashed line) (Permission from John Wiley and Sons; British Journal of Pharmacology 2010;70(4):597–603)
Absorption
Oral medication for a child who requires acute care (e.g., in shock) is uncommon because there is decreased absorption and delayed drug response from reduced perfusion to the gastrointestinal tract. Nevertheless, it is an easy and effective route for many drugs. Enzymes present in the liver and gastrointestinal tract can metabolize drugs before they reach the systemic circulation. This is known as the “first-pass effect”. When a drug has a significant first-pass effect, a smaller quantity (less than 100 %) of the orally administered dose reaches the site of action. Thus, the drug is said to have low bioavailability. Bioavailability becomes 100 % for intravenously administered drugs because the whole dose is given into the systemic circulation. Numerous factors influence absorption via the oral route: gastric emptying, gastric acidity, intestinal motility, bacterial colonization and enzymatic function (see Chap. 3). This is especially important in the first 2 years of life.
In neonates, gastric emptying is delayed which is reflected in a longer interval to reach a lower peak plasma concentration. Gastric acidity is important for drug ionization and absorption. Gastric acidity is decreased in neonates. The more alkaline environment favors the absorption of drugs that are destroyed in acidic media (such as ampicillin) but reduces the uptake of acidic drugs (such as phenobarbitone).
The rectal route for drug administration is undesirable in an acute setting because of the slow and unpredictable absorption of drugs. Despite this, it is an effective method to sedate children with diazepam rectal suppositories. Similarly, intramuscular (IM) injection of medication in children is discouraged. Unpredictable absorption may arise in a critically ill child with hypotension and reduced perfusion to the muscles. Besides, IM injection is painful.
In older children, fentanyl and clonidine transdermal patches offer another route for drug administration. Absorption can be increased in neonates and preterm babies because of the thin skin and better hydration (larger surface area versus body weight). Caution is necessary as there are reports of toxicities following the topical application of medications (e.g., with epinephrine, salicylic acid).
Medications that reach the systemic circulation are, to a certain extent, protein-bound. Infants have decreased protein binding of drugs. Several factors are responsible for this: fetal albumin has decreased binding affinity, reduced amounts of binding proteins (e.g., albumin) are available and there is competition by other agents for the protein binding sites, such as bilirubin or hormones that have crossed the placenta.
Diseases that contribute to hypoalbuminemia, acidosis and hyperbilirubinemia may reduce the protein binding leading to more unbound drug to occupy active sites. Phenytoin is highly protein bound. Clinicians need to identify any altered protein binding so that a measurement of the free or unbound concentration can be determined to better assess therapy.
The majority of medications are administered intravenously to acutely sick children. The pediatric daily maintenance fluid volume averages 1,500 ml/m2/day. It is necessary to plan an intravenous fluid management strategy, taking into account the rate of infusion and the small volumes required. High concentrations of drugs have adverse effects (e.g., phlebitis) on the smaller veins. Clinicians are encouraged to follow recommendations regarding the maximum concentration and the infusion regime for potent drugs with a short half-life such as norepinephrine. Dilution of drugs should be double-checked by a senior staff before the continuous intravenous infusion of medication is commenced, e.g., mcg/kg/min. Children with cardiac or renal disorders, who are managed on low fluid regime, require attention to fluid balance.
Distribution
Dug distribution following administration into the systemic circulation is affected by physicochemical characteristics of the drugs and the pathophysiological status of the child. In particular, age-related altered body composition (e.g., in preterm neonate, obese child) and the dynamic changes in the critical clinical condition (e.g., shock, tissue perfusion), are special factors that should be considered (see Chap. 4).
When expressed as a percentage of body weight, total body water (TBW) is greater in the newborn and decreases with increasing age (full-term newborn – 75 %, 3 month old – 60 % and adult – 55 %). Extracellular water as a percentage of body weight is 50 % in premature infants, 35 % in infants and 19 % in adults. The higher TBW and extracellular water in infants result in a large volume of distribution (Vd) for water-soluble drugs. Vd is important for calculating the loading dose (first dose), where
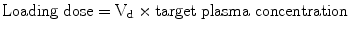 For example, the loading dose of the water-soluble drug gentamicin is larger in infants versus older children. In contrast, neonates require a smaller loading dose of fat soluble drugs (e.g., diazepam).
For example, the loading dose of the water-soluble drug gentamicin is larger in infants versus older children. In contrast, neonates require a smaller loading dose of fat soluble drugs (e.g., diazepam).
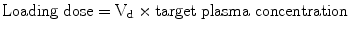
The differing proportions of fat in the various categories of children need to be taken into consideration. A preterm has 1–2 % fat tissue versus 15 % in a term newborn.
Metabolism
The liver is the major organ for drug metabolism. The process of metabolism inactivates lipid-soluble drugs to water-soluble compounds (polar) for excretion. Metabolized compounds can be an active metabolite (e.g., normeperidine) that contributes to pharmacological effect as well as to toxicities when it accumulates in children with poor renal function.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


