13 The oesophagus, stomach and duodenum
Surgical anatomy
Oesophagus
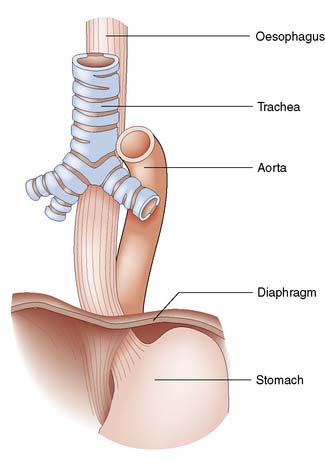
The oesophagus extends from the cricoid cartilage (at the level of vertebra C6) to the gastric cardia and is 25 cm long. It has cervical, thoracic and abdominal portions. The oesophagus passes through the diaphragm at the level of the 10th thoracic vertebra and the final 2–4 cm lie within the peritoneal cavity. The relationships are shown in Figure 13.1.
Stomach and duodenum
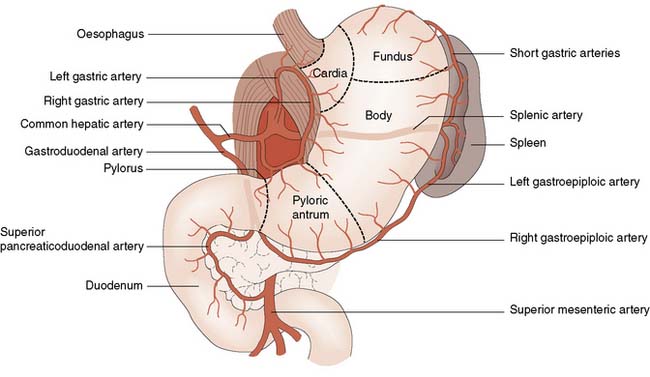
The stomach has an extensive blood supply (Fig. 13.2) derived from the coeliac axis. When the stomach is used as a conduit in the chest, as in an oesophagectomy, the left gastric, left gastroepiploic and short gastric vessels are divided, and the stomach then relies on the right gastric and right gastroepiploic vessels for viability. Ischaemia does not usually result because of the free communication between the vessels supplying the stomach. The blood supply to the duodenum is derived from both the coeliac axis (via the gastroduodenal artery) and branches from the superior mesenteric artery. The veins from the stomach and the duodenum accompany the arteries and drain into the portal venous system.
Surgical physiology
Gastric secretions
Classically, gastric secretion has been divided into three phases:
• Cephalic (neural) phase. Signals arise in the central cortex or appetite centres, triggered by the sight, smell, taste and thought of food, and travel down the vagus nerves to the stomach
• Gastric phase. Food (in particular protein digestion products) causes the release of acid, this release controlled by a negative feedback mechanism dependent upon the pH of the stomach. The gastric phase accounts for the greatest part of daily secretion, approximately 1.5 litres
• Intestinal phase. The presence of food in the duodenum triggers the release of a number of hormones, including duodenal gastrin. These exert a positive feedback effect on the stomach, causing a small increase in gastric secretion.
History and symptoms
Dysphagia
• Onset. Sudden onset suggests a foreign body. In carcinoma, the dysphagia occurs over a period of weeks, whereas in achalasia and benign strictures, symptoms tend to develop over a number of years
• Site. The actual site of obstruction correlates poorly in general to where the patient feels the discomfort, although some patients who feel the obstruction to be high may have a pharyngeal pouch
• Progression. Dysphagia due to an oesophageal stricture (benign or malignant) tends to be progressive whereas patients with motility disorders will often have intermittent symptoms.
• Severity. Difficulty in swallowing solids is initially typical of carcinoma, whereas achalasia and other motility disorders tends to be associated with dysphagia to liquids as well
• Causes. A list of the common causes of dysphagia is shown in Table 13.1.
Table 13.1 Causes of dysphagia
| Intraluminal | Intramural | Extrinsic |
|---|---|---|
| Pharynx/upper oesophagus | ||
| Foreign body | Pharyngitis/tonsillitis Moniliasis Sideropenic web Corrosives Carcinoma Myasthenia gravis Bulbar palsy | Thyroid enlargement Pharyngeal pouch |
| Body of oesophagus | ||
| Foreign body | Corrosives Peptic oesophagitis Carcinoma | Mediastinal lymph nodes Aortic aneurysm |
| Lower oesophagus | ||
| Foreign body | Corrosives Peptic oesophagitis Carcinoma Diffuse oesophageal spasm Systemic sclerosis Achalasia Post-vagotomy | Para-oesophageal hernia |
Dyspepsia
Dyspepsia is something of a ‘catch all’ term used to describe the symptoms of indigestion. Patients may have some or all of the following; epigastric pain, belching, heartburn, nausea, early satiety or reduced appetite. These symptoms are very common in the general population. Current guidance from the National Institute for Clinical Excellence (NICE) in the UK, recommends lifestyle advice, medication review and empirical treatment for the majority of patients with dyspepsia but without so-called alarm symptoms (weight loss, progressive dysphagia, iron deficiency anaemia, epigastric mass and persistent vomiting) (EBM 13.1). Unfortunately, the symptoms of early upper GI malignancy are very similar to dyspepsia and only advanced malignancies tend to cause alarm symptoms. Patients with advanced upper GI malignancy have a poor prognosis despite aggressive therapy, which creates a dilemma; which patients with dyspepsia should be referred for endoscopy? NICE guidance on dyspepsia should be applied with caution and doctors should have a low threshold for endoscopy in any patient who does not improve quickly with simple treatment. It is also imperative that a careful history is taken and anaemia excluded.
Examination
Physical examination might reveal signs that will aid in the diagnosis of upper GI disorders. A smooth tongue, pallor and koilonychia are signs of iron deficiency anaemia, which can be present in oesophageal carcinoma, oesophagitis and Plummer–Vinson syndrome (see p. 176).
Investigations
Chest X-ray
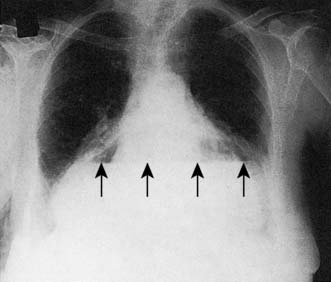
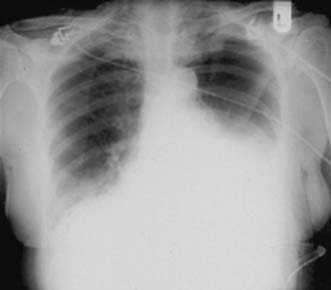
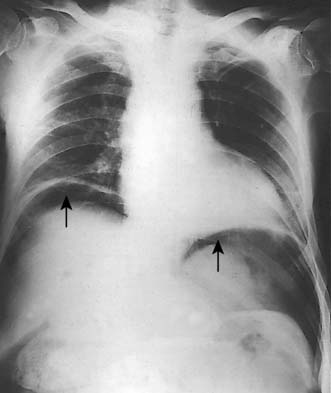
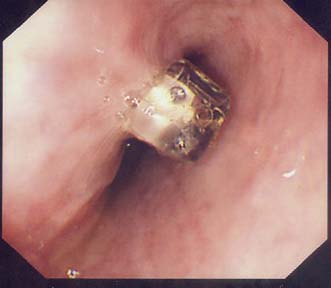
A simple chest X-ray may show any of the following signs: pulmonary consolidation and fibrosis following aspiration in patients with oesophageal motility disorders and oesophageal carcinoma, an air-fluid level behind the heart shadow from a large hiatus hernia with intrathoracic stomach (Fig. 13.3), a mediastinal mass of lymph nodes and pulmonary metastases in oesophagogastric cancer, air in the mediastinum and neck after perforation of the oesophagus (Fig. 13.4) or under the diaphragm from a perforated peptic ulcer (Fig.13.5).
Contrast swallow/meal
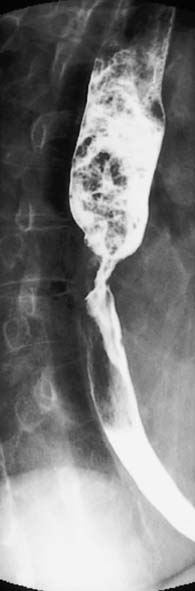
A contrast swallow/meal using barium liquid or water soluble contrast may be useful:
• As a primary investigation when access to endoscopy is limited (Fig. 13.6)
• In a very frail patient with dysphagia or vomiting who might not be deemed fit enough for endoscopy (rare)
• To exclude a pharyngeal pouch prior to endoscopy
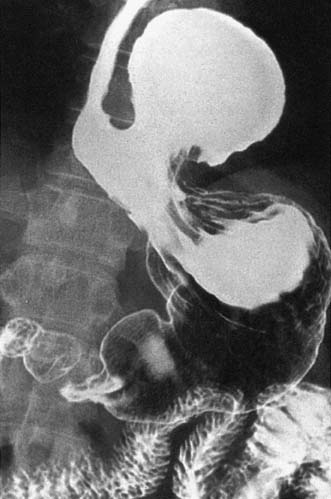
• To complement endoscopy and provide additional anatomical information (i.e. for patients with a large hiatus hernia) (Fig. 13.7)
Endoluminal ultrasound
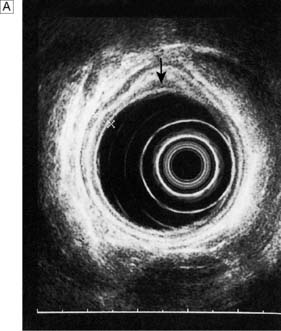
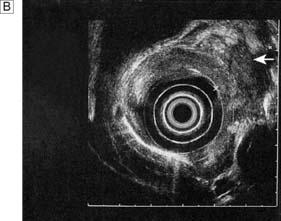
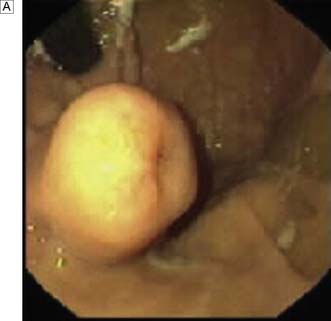
Endoscopic ultrasonography (EUS) uses a variety of endoscopes containing high frequency ultrasound probes at their tips to investigate patients with upper GI disorders. By placing the probe within the GI lumen great detail of the underlying structures can be obtained. EUS is mostly used for staging the ‘T’ and ‘N’ component of TNM staging for oesophagogastric cancer (Fig. 13.8). In addition, EUS is increasingly being used to allow fine needle biopsy of suspicious lymph nodes, especially when the status (positive or negative for cancer) will have profound implications for the intention of a patient’s treatment (curative or palliative). EUS is also useful for investigating submucosal lesions of the stomach such as gastrointestinal stromal tumours (GISTs) (Fig. 13.9) (see p. 176).
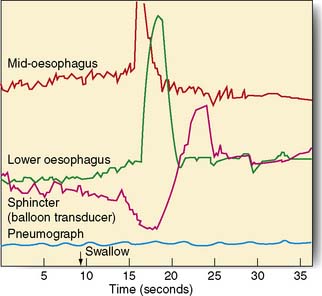
Manometry and pH studies
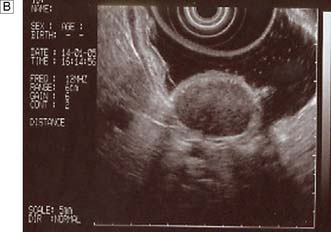
Measurements of lower oesophageal pH can be made over a 24-hour period using an intraluminal electrode placed 5 cm proximal to the lower oesophageal sphincter attached to a catheter passed through the nose and pharynx. Alternatively, oesophageal pH can be measured over three days using a Bravo capsule® which is clipped to the oesophageal mucosa and is wireless (Fig. 13.10). Patients can indicate symptom events during the recording and these can be correlated with the pH trace. A composite scoring system (the DeMeester score) is then used to diagnose pathological GORD. Oesophageal pressure and peristalsis can also be analysed during a series of swallows (station manometry) or over a longer period (ambulatory manometry) (Fig. 13.11). To diagnose gastro-oesophageal bile reflux in patients with duodenogastric reflux a Bilitec probe® is used.
Diagnosis and management – oesophagus
Gastro-oesophageal reflux disease (GORD) and Barrett’s oesophagus
• a physiological high-pressure zone (not a true sphincter) in the lower end of the oesophagus
• the mucosal rosette at the cardia, which acts like a plug
• the angle at which the oesophagus joins the stomach between the left border of the oesophagus and the fundus (angle of His)
• the diaphragmatic sling (crura), which acts like a pinchcock at the lower end of the oesophagus
• the high-pressure area at the lower end of the oesophagus, caused by the positive intra-abdominal pressure.
Diagnosis and management
Barrett’s oesophagus is a histological diagnosis made after endoscopic biopsies. GORD can cause oesophagitis and in some patients this leads to a metaplastic change in the mucosa from squamous to columnar type. Barrett’s oesophagus is of interest as it can become dysplastic which in turn can lead to oesophageal adenocarcinoma. This disease is increasing in incidence and Barrett’s patients offer a target group for surveillance to detect early neoplastic disease (EBM 13.2).
Anti-reflux surgery
Although surgical treatment of patients with severe anti-reflux disease has always been associated with good long-term outcomes, it has taken the introduction and refinements of laparoscopic techniques to bring the surgical option to more patients. The indications for surgery include those whose symptoms cannot be controlled by medical therapy, those with recurrent strictures despite treatment, and young patients who do not wish to continue taking acid suppression therapy for several decades. Symptoms that fail to be brought under control with acid suppression therapy are usually due to high-volume alkaline reflux, and surgery is an extremely effective cure (EBM 13.3). The presence of Barrett’s metaplasia alone is not considered a suitable indication for anti-reflux surgery.
13.3 Surgery for gastro-oesophageal reflux disease
For further information: www.acg.gi.org/physicians/guidelines/GERDTreatment.pdf.
Surgery involves reduction of the hiatus hernia if present, approximation of the crura around the lower oesophagus, and some form of fundoplication. This takes the form of mobilizing the fundus of the stomach from its attachments to the undersurface of the left hemidiaphragm and the left crus, and then wrapping it around the oesophagus, either anteriorly or posteriorly. The most common procedure currently performed is the Nissen fundoplication, in which the fundus is taken posteriorly around the lower oesophagus and sutured to the left anterior surface of the left side of the proximal stomach as a 360° wrap (Fig. 13.12). Other procedures involving a partial (incomplete) fundoplication include the Toupet (posterior 270° wrap) and the Watson (anterior 180° wrap) repairs. Current data do not demonstrate much difference between the various approaches although early postoperative dysphagia is commoner with 360° wraps compared to the partial wraps. All procedures have a success rate in curing the symptoms of reflux of around 90% at a year and 70–80% at 10 years. Unwanted complications after surgery include gas bloat (inability to belch), dysphagia, early satiety and increased flatus. These operations are now carried out laparoscopically, with excellent results in skilled hands.
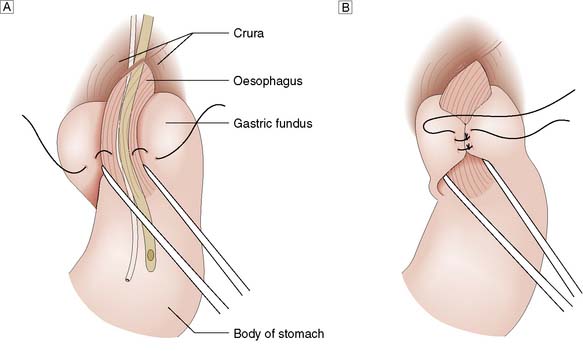
Fig. 13.12 Fundoplication for GORD.
A Gastric fundus wrapped around the lower oesophagus.B Fundal wrap sutured in position.
Summary Box 13.1 Gastro-oesophageal reflux disease
• Reflux type symptoms are very common
• ‘Life style’ advice to patients is important (i.e. smoking, dieting etc)
• Proton pump inhibitors are generally effective treatment
• GORD for > 10 years (especially men) is a risk factor for Barrett’s oesophagus
• Screening and surveillance for Barrett’s patients increasingly relevant
• Laparoscopic anti-reflux surgery is clinically effective and cost efficient.
Hiatus hernia
A hiatus hernia is an abnormal protrusion of the stomach through the oesophageal diaphragmatic hiatus into the thorax. There are two types, sliding (90%) and rolling (10%) (Fig. 13.13). A sliding hernia occurs when the stomach slides through the diaphragmatic hiatus, so that the gastro-oesophageal junction lies within the chest cavity. It is covered anteriorly by peritoneum, and posteriorly is extraperitoneal. A rolling or para-oesophageal hernia is formed when the stomach rolls up anteriorly through the hiatus; the cardia remains in its normal position and therefore the cardio-oesophageal sphincter remains intact.
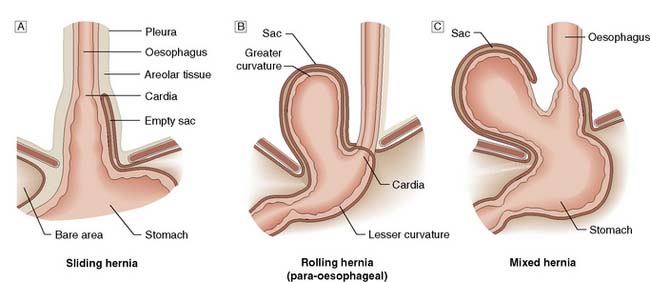
Fig. 13.13 Types of hiatus hernia.
A Sliding hernia.B Rolling hernia (para-oesophageal).C Mixed hernia.
Clinical features
Hiatus hernias are often asymptomatic, but can produce some of or all the following symptoms:
• Heartburn and regurgitation owing to an incompetent lower oesophageal sphincter, which is aggravated by stooping and lying flat at night, and can be relieved by antacids.
• Oesophagitis resulting from persistent acid reflux, which leads to ulceration, bleeding with anaemia, fibrosis and stricture formation.
• Epigastric and lower chest pain, especially in para-oesophageal hernias, as the herniated part of the stomach (usually the fundus) becomes trapped in the hiatus. This can be a surgical emergency owing to the obstruction and strangulation of the stomach.
• Palpitations and hiccups, symptoms caused by the mass effect of the hernia in the thoracic cavity irritating the pericardium and the diaphragm. In patients with a large rolling hiatus hernia, displacement of the whole stomach may result in a volvulus into the chest, producing symptoms of vomiting from gastric outflow obstruction.