The Normal Endometrium
Components of the Normal Endometrium 290
Endometrium During the 28 Day Idealized Normal Menstrual Cycle
Menstrual Endometrium (Days 1–3 of 28)
Proliferative Phase (Days 4–15 of 28)
Interval Endometrium (Day 16 of 28)
Secretory Endometrium (Days 17–28 of 28)
Early Secretory Phase, Days 16–18: Changing Vacuolar Patterns
Mid-secretory Phase, Days 19–21: Increasing Stromal Edema
Methods of Endometrial Sampling
Problems in Interpretation of Endometrial Specimens
Components of the Normal Endometrium
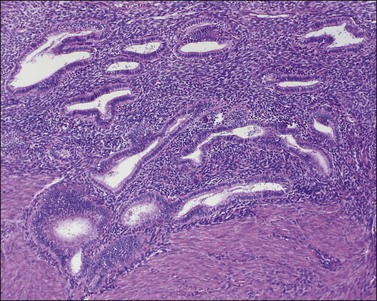
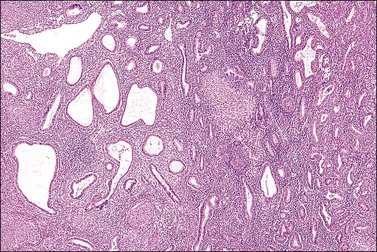
The endometrium merges with the mucosa of the fallopian tube at its upper extreme and with the endocervical epithelium at its lower end. The junction with the fallopian tube epithelium is usually abrupt, although the exact position may vary considerably. Uncommonly, endometrium may line the tube some centimeters lateral to the cornu, a condition referred to as ‘endometrialization’ (to be distinguished from endometriosis, Chapter 22). At the junction of the endometrium with the endocervical epithelium, however, there is a gradual transition from one type of mucosa to the other, sometimes over a distance of as much as 1 cm. This is the lower uterine segment, which contains glands with features between those of the endometrium proper and the endocervix (Figure 14.1). Not uncommonly, endometrial glands will be found deep to the endocervical lining. Glands of the lower uterine segment show practically none of the morphologic effects that hormonal stimulation elicits in the fundus. Care must be taken to recognize this tissue for what it is, so that the inactivity in these pieces is not taken to mean that the endometrium as a whole is not being stimulated or is not responding. Glands in this area are prone to be partly lined by epithelium containing a mixture of undistinguished columnar cells admixed with ciliated cells. The stroma of the lower uterine segment differs from that of the endometrium proper in being rather more fibrous and displaying cells that are generally more spindled. The glands are typically flattened and slit-like and the epithelial cells lack mucus.
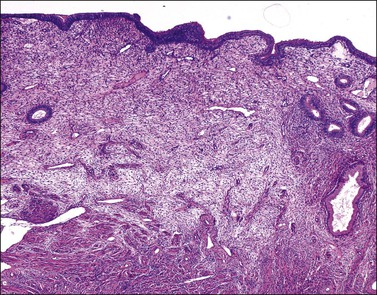
Figure 14.1 Lower uterine segment mucosa, or ‘isthmic endometrium.’ In a hysterectomy specimen, the isthmic endometrium is seen as a zone of poorly developed glands in fibrous stroma, which lack the cyclical hormonally induced changes of endometrium within the uterine corpus.
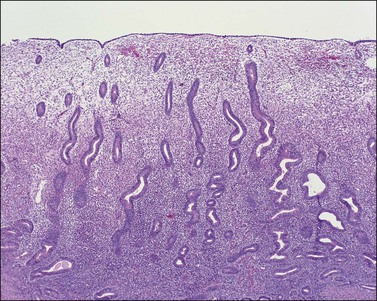
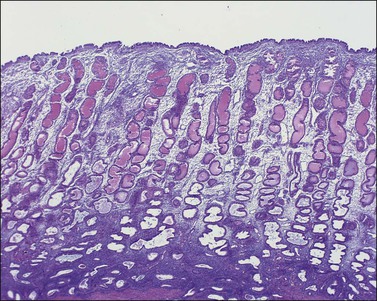
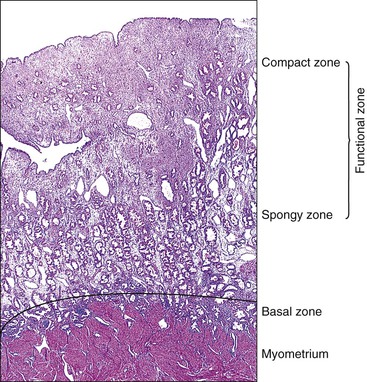
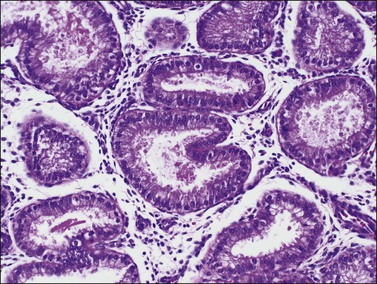
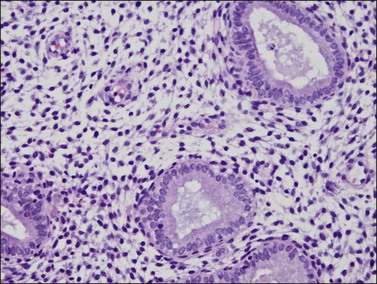
The endometrium from the uterine body and fundus is generally fairly uniform from one area to another. There is, however, variation within the endometrial thickness depending on the vertical position of the tissue in relation to the surface epithelium and the endometrial–myometrial junction (Figure 14.2). These layers become more pronounced as the menstrual cycle progresses (Figure 14.3). The basal layer (stratum basalis) is adjacent to the myometrium, and consists of tubular glands, occasionally branching, lined by simple to pseudostratified epithelium in a more basophilic, compact stroma (Figure 14.4). The glandular epithelium shows no evidence of secretory activity whatever the phase of the cycle, and there is no or minimal mitotic activity in either glands or stroma. As the overall volume of glands is small compared with that in the functional layer, the stroma is relatively more prominent. The stroma also appears more cellular as it is composed of largely spindled nuclei with only scant, inapparent cytoplasm.
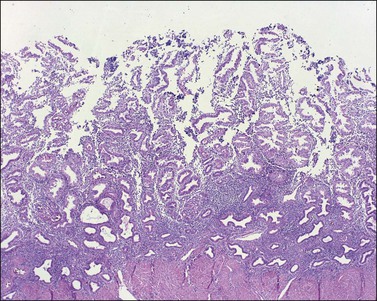
The remaining endometrium is the functional zone (stratum functionalis), which is further subdivided into the superficial compact layer (stratum compactum) and the deeper spongy layer (stratum spongiosum). This distinction only becomes striking in the late secretory (postovulatory or luteal) phase (Figure 14.5). At that time the spongy layer consists of glands showing maximal secretory activity but a relatively unresponsive stroma that does not develop a good predecidual response apart from the immediate vicinity of the spiral arterioles. Stroma in the more superficial compact layer, on the other hand, responds remarkably to hormonal stimulation with a prominent predecidual reaction and numerous granulated lymphocytes (see later). Glands in this zone are stretched thin by the expanding stroma, and demonstrate less secretory activity. It is apparent therefore that the morphology of the endometrial stromal and glandular cells is a function not only of the systemic hormonal environment, but also of the position in the corpus or lower uterine segment, and vertical location within the endometrial layers. As material from all of these layers routinely appears in curettings, the pathologist must be aware of characteristic appearances at all sites throughout the menstrual cycle.
Surface Epithelium
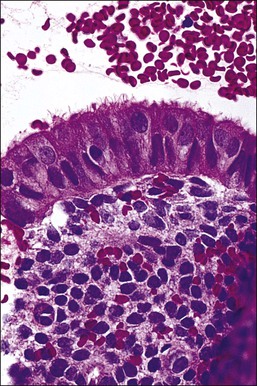
The surface epithelium of the endometrium is continuous with the glandular epithelium and is generally similar. However, the constituent cells show less marked cyclical variation than the cells in the glands (Figure 14.6), responding relatively weakly to circulating sex steroids, and are frequently ciliated. Although subnuclear vacuolation and mitotic activity are seen, these features do not always accurately reflect the time of the cycle. There is usually no problem in identifying surface epithelium in curettings, but the few small epithelial strips that may constitute an entire biopsy sample in an atrophic endometrium can be difficult if not impossible to characterize with any degree of certainty.
Glandular Cells
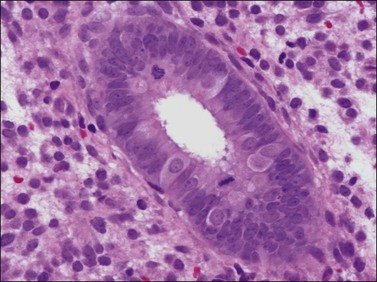
There are three types of endometrial glandular cells: the secretory cell (Figure 14.7), the ciliated cell (Figure 14.8), and the clear cell (Figure 14.9).
• The secretory cells are by far the most abundant and their morphology varies with the time of the menstrual cycle. The various appearances will be covered when the phases of the cycle are described.
• The ciliated cells are more frequent near the cornua and toward the endocervix as well as being quite common in the surface epithelium. Although a normal constituent of the endometrium, the ciliated cells are particularly under the influence of estrogens and become more prominent in conditions of estrogen excess (e.g., anovulatory cycles).1 As ciliated cells are so common, they must be considered as normal and not as ‘ciliated metaplasia’ as described elsewhere in the literature. Furthermore, in specimens, where only strips of cells are produced, the presence of cilia is useful to suspect that an estrogenic milieu is present whether from endogenous or exogenous sources and that the specimen is not atrophic.
• The clear cells are much less common and are thought to be precursors of the ciliated cells. They are most frequently seen in the proliferative phase.
Stromal Cells
The morphology of the endometrial stromal cells varies dramatically throughout the menstrual cycle. During the proliferative phase, the stromal cells are small and mostly compact, with oval, hyperchromatic nuclei and inapparent cytoplasm. In the mid-proliferative phase, at the preovulatory peak of serum estrogen levels, stromal cells are separated by increased intercellular edema. At the end of the proliferative phase the nuclei become slightly larger and their chromatin a little less dense (Figure 14.10). The stroma again becomes edematous in the middle of the secretory phase, reaching a peak at about the 22nd day of a normalized 28 day cycle (again due to an estrogen peak), after which the cells of the compact zone progressively undergo predecidual change, developing into polygonal cells with vesicular nuclei and abundant, pale cytoplasm with well-defined cell borders (Figure 14.11) The terms ‘predecidua’ and ‘pseudodecidua’ are often used to describe this change in the morphology of the stromal cells as a response to endogenous and exogenous progesterone, respectively. The term ‘decidua’ is properly reserved for the change seen in pregnancy, but in practice many pathologists use the terms interchangeably. While it is true that the morphologic changes in the cells are qualitatively the same whether it has been brought about by physiologic levels of progesterone or by synthetic progestins (as in oral contraceptives), there usually is a quantitative difference in both the amount of cytoplasm present in any given cell and the proportion of stromal cells with the change. In general, decidual change is more extensive and uniform in pregnancy and in patients receiving exogenous progestins (Figure 14.12), whereas in premenstrual endometrium a significant proportion of the cells are only minimally or partially affected (compare with Figure 14.11). Decidual change is first apparent adjacent to the spiral arterioles but toward the end of the secretory phase and in pregnancy the change becomes diffuse. The stromal cells that are situated deeper in the endometrium, lying between the active glands of the spongy layer, show little, if any, decidual change and remain fairly nondescript (Figure 14.13). Occasionally, small bundles of smooth muscle may be found in the endometrial stroma (Figure 14.14). The decidual cells of the endometrial stroma, together with an exponential increase in granulated lymphocytes and natural killer (NK) cells, and the synthesis of a variety of cytokines and extracellular matrix proteins including laminin and fibronectin play a central role in the process of nidation (see Chapter 32).
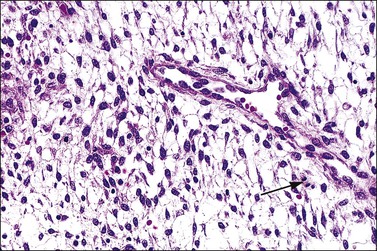
Figure 14.10 Endometrial stroma. Proliferative phase. Stromal mitotic activity (arrow) is seen. Part of a spiral arteriole is present.
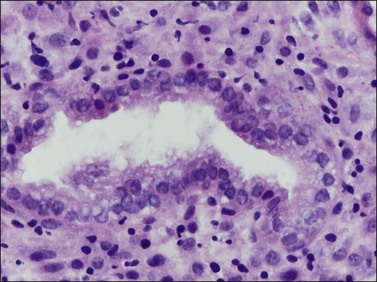
Figure 14.11 Endometrial stroma. Late secretory phase (day 27), compact layer. The stromal cells surrounding the gland show predecidual change and have abundant cytoplasm. Granulated lymphocytes are present.
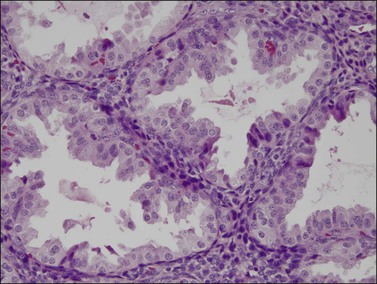
Figure 14.13 Endometrial stroma. Late secretory phase (day 27), spongy layer. The glands are active but the stromal cells are small with no decidual change.
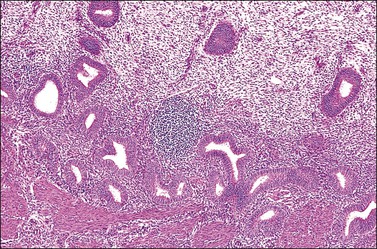
Endometrial Lymphocytes
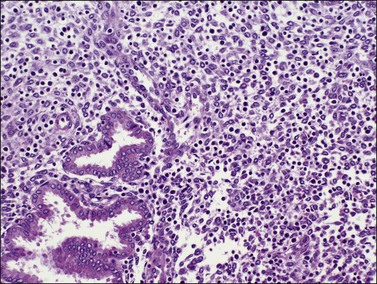
Several subpopulations of T-lymphocytes reside in the normal endometrium, and small lymphoid aggregates are normal, particularly in the basal zone (Figure 14.15). CD4+ and CD8+ T-cells are randomly scattered throughout the functional layer and, in the absence of an inflammatory process, show only a modest variation in density throughout the menstrual cycle, increasing toward menstruation.2 In any form of endometritis, they aggregate near and around glands and can be seen within the gland lumens along with CD68+ or CD163+ macrophages. Macrophages are normally found in all areas of the functional and basal zones.
In contrast, small, rounded cells with hyperchromatic nuclei that are usually kidney-shaped or segmented increase in number in the endometrial stroma in the second half of the cycle (Figures 14.16 and 14.17). These cells are known as endometrial granulocytes, endometrial granular cells, or K-cells. The cytoplasm contains eosinophilic granules of variable size, so that the cells are also often mistaken for infiltrating polymorphonuclear leukocytes, particularly in the presence of early or imminent menstrual fragmentation (neutrophils are not seen in a normal endometrium until menstruation is well established and their presence at other times of the cycle indicates inflammation). These cells are large granulated lymphocytes that, on flow cytometric analysis, exhibit the unusual T-cell phenotype CD56+, CD3−, CD16−, and also have an NK function. Endometrial NK cells increase in number dramatically in the late secretory phase of the menstrual cycle, sometimes reaching a population density exceeding 25% of all stromal cells. If conception and implantation occur, they continue to increase in number and make up about 70% of the stromal lymphocytes in the first trimester of pregnancy. Endometrial and decidual NK cells are distinct from other classes of NK cells, having both a major immune regulatory function and a critical role in remodeling of the endometrium after implantation.2,3 Our expanding understanding of the important role of decidual NK cells is leading to the possibility of infertility therapies aimed at disorders in their function.4
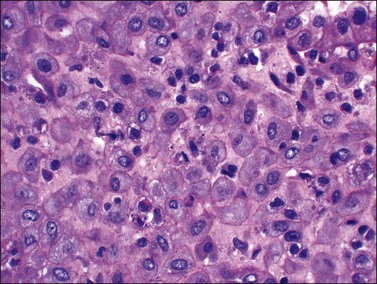
Figure 14.17 Large granular lymphocytes in predecidua of normal late secretory endometrium. The cells have hyperchromatic, kidney-shaped nuclei and eosinophilic granules in the cytoplasm.
In comparison to T-lymphocytes, B-lymphocytes are normally sparse in the endometrium. Whether rare plasma cells can be seen in normal endometrium remains a controversial topic, as observations differ according to the population studied and detection method employed. One of the few studies to rigorously select normal women used a sensitive methyl-green pyronine stain to identify small numbers of plasma cells in about one-third of normal endometria.5
Blood Vessels
The arterial supply of the endometrium is from the radial arteries that arise from the arcuate arteries in the myometrium. The radial arteries branch near the endometrial–myometrial interface, forming the basal arteries. As these ascend through the functionalis to the endometrial surface, they become the spiral arterioles (Figure 14.18). The spiral arterioles respond to the varying levels of ovarian hormones and become prominent in the second half of the secretory phase, under the influence of progesterone. Coiling becomes most pronounced when the stromal edema is reabsorbed prior to menstruation. In the proliferative phase, the arterioles show little coiling and are confined to the deeper levels of the functionalis. There is an irregular network of venous channels with the veins frequently intersecting, forming venous lakes. Lymphatic vessels are present in normal endometrium, but disappear in decidualized endometrium during pregnancy.6
Endometrium during the 28 Day Idealized Normal Menstrual Cycle
The endometrium undergoes a complex and orchestrated series of changes during menstrual cycle progression that when properly interpreted can provide useful information about the patient’s hormonal state.7–11 The main features throughout the cycle are summarized and illustrated in Figure 14.19. The endometrial cycle is renewed with menses, and then customarily divided into two sequential phases, the proliferative (preovulatory or follicular) and the secretory (postovulatory or luteal) phases. This division of the cycle is related, of course, to the hormones stimulating it, with estrogen predominating in the proliferative phase and progesterone in the secretory phase. The endometrial cycle is often standardized in pathology reports as an idealized 28 day cycle beginning on the first day of clinical menses, followed by a proliferative phase with ovulation on day 14. Physiologic differences in total cycle length between women are generally caused by variation in the duration of menses and the proliferative phase, as the postovulatory temporal sequence is a remarkably consistent 14 days. In dating the endometrium it is important to remember that there is a delay of 2–3 days between ovulation and appearance of diagnostic postovulatory histologic changes. This is caused by progressively rising progesterone levels and time necessary for the endometrium to respond. Thus, although clinical ovulation is assumed to occur on day 14, there is a brief ‘interval phase’ corresponding to days 15–16, before definitive progestational effects are evident.
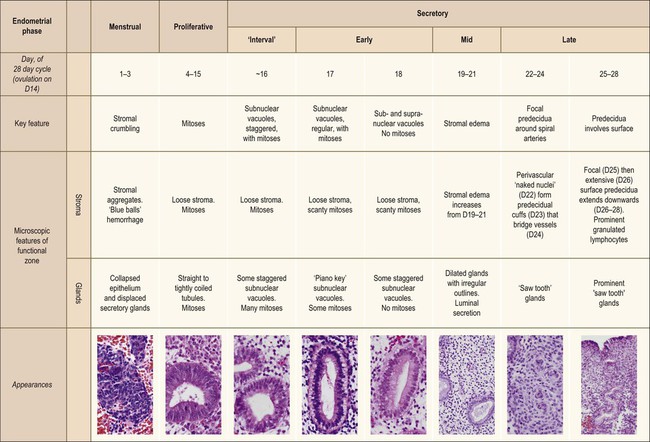
Figure 14.19 Main histologic features of the menstrual cycle. Histologic classification stages, according to 28 day idealized cycle in which day 1 is the first day of clinical menses and ovulation occurs on day 14.
Menstrual Endometrium (Days 1–3 of 28)
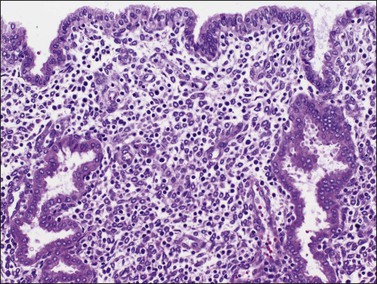
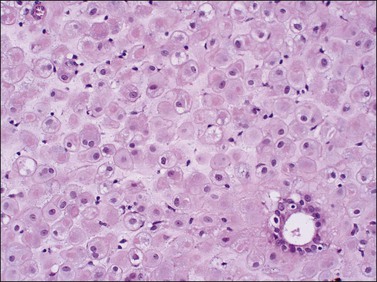
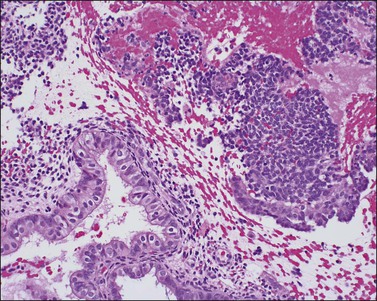
If conception and implantation have not occurred by day 24 (postovulatory day 10), the corpus luteum involutes, leading to a marked fall in progesterone output. The late secretory phase then leads inevitably to the menstrual phase, which defines cycle day 1. This is recognized histologically by very fine crumbling or fragmentation of the stroma and glandular collapse (Figure 14.20), with abundant blood and neutrophils in the background. Two very characteristic changes, especially when seen in combination, are compact balls of stromal cells which, with hematoxylin, have an unusually deep blue color (Figure 14.21), and which are covered by a layer of epithelial cells that appear eosinophilic and reactive. A third change, often seen, is the presence of plump epithelial glandular cells that are frequently multilayered, the so-called ‘papillary syncytial metaplasia’ (Figure 14.22).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree