(1)
University of South Australia, Adelaide, Australia
Abstract
If a new drug’s incremental price-effectiveness ratio (IPER) is above the health shadow price, β c , the best alternative strategy to new drug reimbursement will result in more health benefits to the population, for the same financial cost. The historic decision threshold (k) in most countries is likely to be significantly higher that the health shadow price. If a regulator chooses to reject a new drug as a consequence of adopting the lower threshold, firms might make the following threat: At IPERs below k, it will not be financially viable to supply most new drugs to this country. The weight of this threat could be significant, particularly when the new drugs have substantial clinical benefit for some patient groups. How should a rational institution respond? In this chapter, this question is first analysed in a conventional decision theoretic (non-strategic) model as the optimal response by regulators to historic evidence of the price of new drugs. Then the question is analysed within a price-effectiveness analysis (PEA) framework. PEA uses an applied game theoretic model that assumes firms act strategically and that the health of the population, not the target patients, is the maximand. I conclude that the decision to reimburse a new drug is best analysed as a game with multiple players who act strategically and where the objective of the Institution is to maximise the population’s health. The second conclusion is that the population health-maximising response to the threat is to maintain a threshold price of 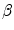 c.
c.
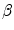 c.
c.8.1 The Reimburser’s Problem
The Reimburser announces that she will pay no more than β c for the additional health effects from new drugs. She estimates β c to be in the order of $5,042 per additional QALY at this time1; only 6.7 % of the max WTP for an additional QALY of $75,000, which is the prevailing maximum acceptable IPER. A group of clinicians approaches the Reimburser with evidence that the majority of the new innovative drugs over the last 10 years were priced at the historic threshold of k (Devlin and Parkin 2004). These clinicians also refer to an excerpt from an article in The New York Times that suggests that society can expect to continue to pay high IPERs for new effective drugs into the future.
Until now, drug makers have typically defended high prices by noting the cost of developing new medicines. But executives at Genentech and its majority owner, Roche, are now using a separate argument—citing the inherent value of life-sustaining therapies. If society wants the benefits, they say, it must be ready to spend more for treatments like Avastin and another of the company’s cancer drugs, Herceptin, which sells for $40,000 a year. “As we look at Avastin and Herceptin pricing, right now the health economics hold up, and therefore I don’t see any reason to be touching them,” said William M. Burns, the chief executive of Roche’s pharmaceutical division and a member of Genentech’s board. “The pressure on society to use strong and good products is there.” (Berenson 2006)
The clinicians argue that these historic and future prices are solid evidence that if the maximum acceptable IPER were lowered, many of the future drugs with significant clinical value of innovation and very high costs would not be available to patients because they would not be reimbursed. Patients will be worse off as a result. The evidence that new drugs tend to be priced at the decision threshold is consistent with the Reimburser’s experience; most new drugs reimbursed in the last 10 years had an IPER substantially above β c and close to the max WTP. Given this evidence of the historic IPER of new drugs, the Reimburser is unsure whether she should enforce β c as the threshold IPER.
The Reimburser asks her Health Economic Adviser two questions:
Will this lower maximum IPER mean that drugs that would otherwise have been reimbursed at prices above $5,042 per QALY will no longer be available to the population at a subsidised price?
If fewer new drugs are available as a consequence of a lowered maximum IPER, will this make the population worse off than it would have been under the existing maximum IPER of $75,000?
The Health Economic Adviser suggests one more question:
How should the rational Institution respond to the following threat:
If the threshold is reduced to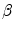 c , less than 15 % of the new drugs that would otherwise be approved will be made available to patients. The population will be worse off as a consequence of a lower threshold price.
c , less than 15 % of the new drugs that would otherwise be approved will be made available to patients. The population will be worse off as a consequence of a lower threshold price.
8.2 A Decision Theoretic Model of the Clinicians’ Case
The problem posed by the clinicians can be represented as a DTM with the following structure: the assumptions, strategies and payoffs that will lead to the prediction that the number of NMEs that are reimbursed will reduce if the threshold IPER reduces.
Such a view of the world is represented in Fig. 8.1 for the (hypothetical) case of the evidence from the previous year of 24 new drug reimbursements, where these new drugs had a proven clinical innovation compared with the best alternative therapy. The (hypothetical) evidence shows that of the 24 NMEs reimbursed in the previous year, only two had an IPER at or below β c = $5,042 per QALY. The clinicians argue that this evidence suggests that only two of the NMEs reimbursed in the previous year would have been subsidised at the maximum acceptable IPER of β c . This situation would represent a loss of 22 NMEs at an average effect of 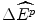 per NME, and hence a loss these patients health effects of 22
per NME, and hence a loss these patients health effects of 22 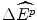 . The clinicians produce further evidence that suggests that the average value of
. The clinicians produce further evidence that suggests that the average value of 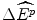 was 850 QALYs per year in the year the drugs were reimbursed.2 Therefore, if these drugs had not been available, around 18,700 QALYs per year in benefits to these patients would not have been experienced.
was 850 QALYs per year in the year the drugs were reimbursed.2 Therefore, if these drugs had not been available, around 18,700 QALYs per year in benefits to these patients would not have been experienced.
 per NME, and hence a loss these patients health effects of 22
per NME, and hence a loss these patients health effects of 22 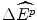 . The clinicians produce further evidence that suggests that the average value of
. The clinicians produce further evidence that suggests that the average value of 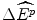 was 850 QALYs per year in the year the drugs were reimbursed.2 Therefore, if these drugs had not been available, around 18,700 QALYs per year in benefits to these patients would not have been experienced.
was 850 QALYs per year in the year the drugs were reimbursed.2 Therefore, if these drugs had not been available, around 18,700 QALYs per year in benefits to these patients would not have been experienced.
Fig. 8.1
The loss in health due to lowered price: a decision analytic perspective
The world within which this DTM resides is supported, implicitly, by three key assumptions.
1.
The budget is assumed to expand to accommodate any purchase at or below the threshold acceptable IPER; it is not fixed and there is no requirement to displace any services to finance the new drug. This is an unconstrained budget in PEA terminology. (See Sect. 3.3)
2.
The payoff to reimbursement is the increase in the health of the target patient group 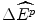 . Therefore, given that there is no displacement and no unfunded “value for money” option (the budget is unconstrained), we can conclude that the population will be worse off by an amount
. Therefore, given that there is no displacement and no unfunded “value for money” option (the budget is unconstrained), we can conclude that the population will be worse off by an amount 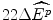 if fewer clinically innovative future drugs are funded than would otherwise be the case.
if fewer clinically innovative future drugs are funded than would otherwise be the case.
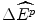 . Therefore, given that there is no displacement and no unfunded “value for money” option (the budget is unconstrained), we can conclude that the population will be worse off by an amount
. Therefore, given that there is no displacement and no unfunded “value for money” option (the budget is unconstrained), we can conclude that the population will be worse off by an amount 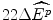 if fewer clinically innovative future drugs are funded than would otherwise be the case.
if fewer clinically innovative future drugs are funded than would otherwise be the case.3.
There is no other price below the offer price at which these firms could produce and sell the drug, that is, there is no lower price where π ≥ 0 (where π is the firm’s economic rent).
These three assumptions lead to a position that is summarised as follows:
1.
No firms will change their offer price as a response to a change in the threshold IPER signalled by the Reimburser;
2.
A reduced threshold IPER will lead to a reduction in the number of NMEs approved by the Reimburser; and
3.
As a consequence, under a lower threshold, the population will be worse off than would otherwise be the case.
The three assumptions that premise the above position are not necessarily applicable to the economic context of reimbursement. First, not all health budgets can be expanded to accommodate all purchases that are below an exogenously-determined threshold IPER. This assumption could be appropriate in some jurisdictions but not in the country of interest. Second, the payoff of an additional NME to target patients is 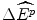 , but the net impact on the population’s health as a consequence of the strategy of Rin a fixed budget is
, but the net impact on the population’s health as a consequence of the strategy of Rin a fixed budget is 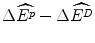 , where
, where 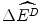 is the average health effects displaced to finance the additional costs of a new drug. There could be a loss in potential health gains
is the average health effects displaced to finance the additional costs of a new drug. There could be a loss in potential health gains 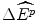 to target patients if a drug that would otherwise be reimbursed is not subsidised. However, the services that would otherwise have been displaced to finance the new drug are no longer displaced. Therefore the reduction in health gains to the target patients would need to be offset by the gain
to target patients if a drug that would otherwise be reimbursed is not subsidised. However, the services that would otherwise have been displaced to finance the new drug are no longer displaced. Therefore the reduction in health gains to the target patients would need to be offset by the gain 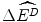 to patients whose services are not displaced. Third, if firms have market power, their current offer price is not necessarily the lowest price at which they would be prepared to produce and sell the drug. Firms with market power can price above the marginal cost of production because there is a lack of competition from other firms willing to increase market share by offering a lower price.3
to patients whose services are not displaced. Third, if firms have market power, their current offer price is not necessarily the lowest price at which they would be prepared to produce and sell the drug. Firms with market power can price above the marginal cost of production because there is a lack of competition from other firms willing to increase market share by offering a lower price.3
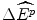 , but the net impact on the population’s health as a consequence of the strategy of Rin a fixed budget is
, but the net impact on the population’s health as a consequence of the strategy of Rin a fixed budget is 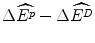 , where
, where 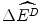 is the average health effects displaced to finance the additional costs of a new drug. There could be a loss in potential health gains
is the average health effects displaced to finance the additional costs of a new drug. There could be a loss in potential health gains 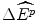 to target patients if a drug that would otherwise be reimbursed is not subsidised. However, the services that would otherwise have been displaced to finance the new drug are no longer displaced. Therefore the reduction in health gains to the target patients would need to be offset by the gain
to target patients if a drug that would otherwise be reimbursed is not subsidised. However, the services that would otherwise have been displaced to finance the new drug are no longer displaced. Therefore the reduction in health gains to the target patients would need to be offset by the gain 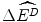 to patients whose services are not displaced. Third, if firms have market power, their current offer price is not necessarily the lowest price at which they would be prepared to produce and sell the drug. Firms with market power can price above the marginal cost of production because there is a lack of competition from other firms willing to increase market share by offering a lower price.3
to patients whose services are not displaced. Third, if firms have market power, their current offer price is not necessarily the lowest price at which they would be prepared to produce and sell the drug. Firms with market power can price above the marginal cost of production because there is a lack of competition from other firms willing to increase market share by offering a lower price.3 The applied economic model developed in the following section accommodates the three characteristics described in the previous paragraph. The first two characteristics are incorporated into the model using a result from the previous two chapters: that the population health payoff to the strategy of reimbursement is the net effect of adoption and displacement. More specifically, the model uses the economic payoff to the reimbursement decision, NEBh R : the net population effect of adoption and displacement net the effect of the best alternative strategy. This ensures that the payoff to reimbursement will identify whether it is the population health-maximising strategy and not simply one with a net population health effect greater than zero. This payoff is analogous to the use of economic rent as the payoff to firms in economic models, rather than accounting profit, and is consistent with the objective of maximising population health. (See Sect. 7.6 for a discussion of the net economic benefit.) The third characteristic, strategic behaviour, is incorporated by using a GTM rather than a DTM. The use of a GTM allows the reimbursement problem to be analysed as a high stakes game, where small changes in the decision threshold can result in a significant change in profits for firms and health for the population.
8.3 The High Stakes Game of New Drug Reimbursement
Firms hold patents for their new drugs. Patents are a policy (legislation) intended to correct for the failure of the market to provide an incentive to invest in R&D. Patents achieve this objective by providing market exclusivity; no other firms can produce the patented item unless they are licensed by the patent holder. However, patents also create market power and therefore the patent holding firm is not necessarily a price taker, unlike a firm in a perfectly competitive market. So economic theory suggests we ask: Why would we assume that a firm with market power will offer the new drug at an IPER substantially below the Reimburser’s threshold when: (1) it knows that the Reimburser will make the same decision (reimburse for the target group) at a higher price; and (2) no other firm will compete and offer the drug at a lower price?4
Unlike the situation in a perfectly competitive market, where at any point in time there is only one price a profit-maximising firm can charge for a given item (the market price), market power means that firms can (and must) select a price strategically. In this case, “strategically” means the monopolist firm makes reference to the expected response by a monopsonist purchaser in the domestic market to possible offer prices. Given the presence of strategic behaviour, the economic model used to characterise the reimbursement process in this section is game theoretic rather than a DTM. How does a GTM differ from a DTM? And why aren’t GTMs used in pharmaco-economics?
8.3.1 Game Theoretic vs. Decision Theoretic Models
Games grow in the spaces where perfect competition does not exist. In perfect competition, the consumers and sellers are price takers and there is no reward for investment in strategic behaviour. In contrast, where the firm and/or the institution have market power, there is a potential for rewards from strategic behaviour. In the case of a new drug, the outcome of the game is the allocation of the economic surplus (value) from clinical innovation between the firm and the institution as agent of consumers. The IPER of the new drug is the mechanism by which this allocation of surplus occurs and a share of this surplus is the possible reward for strategic behaviour. The decision threshold signalled by the institution is a key piece of information (and decision) which, if varied, can change the outcome of the game (the allocation of the innovative surplus) by changing the equilibrium price.
There are two elements common to DTMs and GTMs: (1) decision and chance nodes; and (2) the outcomes of these nodes. However, there is a fundamental difference in the structure and solutions of DTMs compared with GTMs: the latter predict the equilibrium set of actions and strategies, given the interactions between the players each with specific preferences, whereas the former predict which strategy will be preferred by a single decision maker, given his or her preferences. Decision analysis can be characterised as the use of a model to select the optimal action of a single decision maker faced with choices with or without uncertainty (chance nodes). In contrast, game theory can be characterised as being concerned with the equilibrium outcomes of strategic interactions of two or more decision makers, with or without uncertainty. Significantly, the equilibrium outcome of a game is not necessarily the optimal outcome for either or both players. In contrast, the lone decision maker in a DTM is selecting the optimal strategy from a number of strategies in order to maximise (or minimise) expected outcomes. There are no other players influencing choice, therefore, the preferred strategy is always implemented. Hence, the result of a DTM is always the selection of the strategy with the best expected outcome for that decision maker.
This fundamental difference is expressed in the structure of the respective models. Both GTMs and DTMs typically have more than one outcome for each decision (for example, cost and effect). The task of decision analysis is for one decision maker to accommodate multiple outcomes, which might need to be traded against each other (for example, additional costs and additional effect). The additional task of game theoretic analysis is to accommodate multiple objective functions (for example, the firm vs. the consumer).
8.3.2 Examples of Published Pharma-Economic Games
There are very few published GTMs of the drug pricing process in either the pharma-economic or the pharmaco-economic literature. Two examples in recent years analyse aspects of the drug reimbursement process (Wright 2004; Antonanzas et al. 2011). A third game analyses the decision by firms to conduct head-to-head comparative trials of their drugs and the role of incentives in changing the predicted no-trial equilibrium outcomes (Mansley et al. 2007). This game is not discussed further in this book but it provides a powerful example of the relationship between expected profit and strategic choices in the design of clinical trials. It is a rigorous game theoretic approach to analysing the question of “why don’t we have more head-to-head clinical trials?”5
Wright (2004) presented the process of drug bargaining in Australia as a five-stage game of complete information. Wright was particularly interested in the welfare implications of regulating the price below the Firm’s6 offer price, bargaining when firms can be differentiated in terms of their quality (high for innovative or low for generic); and a single drug having differential impacts in two patient groups. Wright’s game identified conditions under which certain firms (high quality firms) could benefit from price regulation. It also identified situations under which leakage7 would not reduce a Regulator’s surplus. Wright structured his model such that the two initial options for the Firm are: (1) to have its price regulated (below what would otherwise be charged) but subsidised to the consumer; or (2) for the price to be unregulated and unsubsidised. There is a trade-off inherent in the decision to approach the Reimburser; maintain unit price or increase sales. This option does not seem to be relevant to the Australian setting where almost all firms request reimbursement for drugs that are prescribed outside the hospital setting. However, it is possible that Wright is referring to a situation where, for a few drugs, the PBAC will not reimburse the drug at the offer price due to lack of evidence of effect or unacceptable cost-effectiveness. In this case the Firm is effectively choosing to not lower the price it sells the drug at and have the price to consumers subsidised. Instead it is choosing to maintain the higher price and sell to a market where the financial barrier to access for a consumer could be high.8 Wright does not refer to the presence of a decision threshold nor does the model include a concept of expressing a drug price as an ICER. He refers to prices per course being equivalent across drugs within the same therapeutic group regardless of their “quality”.
Wright’s model is a reminder that drug price is endogenous not exogenous to the decision process. He also points out that there is an opportunity for firms to use lobbying to extract more of the surplus associated with the reimbursement of the drug, once the firm has agreed to a regulated price. This lobbying for higher prices occurs even though the “high quality firm” in Wright’s model is better off by accepting the regulated price in order to gain market share through subsidised drug prices for consumers than by a choosing higher price with no consumer subsidy. Wright concludes that Pharma’s “hostility” to regulation and claim that it reduces profit is a strategy to “extract more of the total surplus generated by regulation.” (p. 810)
However, a second conclusion is less useful in the context of a reimbursement process that uses economic evaluation, decision thresholds and ICERs. Wright observes that the use of regulation has the objective of improving equity however, it also has efficiency implications. He argues that because regulation results in a single price across drugs with varying quality but in the same therapeutic group, these efficiency implications are not desirable.
In fact, groups of drugs with a single price per course and in the same drug class are typically all generics (or all on patent). If a drug is of higher quality (which is assumed to mean more effective than a comparator) and is on patent, then it will have a higher price per course if it prices at an ICER above zero.9 Furthermore, improving equity (in access by consumers through a co-payment scheme) is a different decision from the maximum price an institution should pay for the incremental health effects of a new drug. Wright’s paper provides some insights but does not assist in the choice of a single threshold for new drugs based on their incremental cost and effect.
For their research on the conditions under which a regulator and a firm would both have an incentive for a risk sharing agreement10 (RSA) for a new drug of uncertain benefit, Antonanzas et al. (2011) characterised two possible contracts between Firms and Regulators: RSAs and non-RSAs. In their GTM of complete information, the stylised RSAs required Regulators to pay Firms only if a patient is cured whereas non-RSAs require payment to the Firm per patient treated, regardless of the observable response by patients. The paper established the conditions under which the preferences of the Firm and the Regulators would be aligned and a contract (either RSA or non-RSA) would be mutually preferable. They found that if drugs have a relatively low cost impact, health funders will prefer not to risk share, and with high cost impact drugs and low costs of monitoring they would prefer an RSA.
8.3.3 Why Aren’t Games Used in Pharmaco-Economic Models?
The pharmaco-economic literature has a rich tradition of DTMs but not GTMs. A possible explanation is that pharmaco-economics occupies the only space in the reimbursement process within which there is no strategic behaviour. This space is the (non-strategic) behaviour of the new molecule given patient characteristics, dose and duration of therapy. All other aspects of the new drug involve strategic behaviour, including the generation of evidence from clinical trials, the construction of pharmaco-economic models to maximise the possible additional benefit and minimise the additional cost; the offer price selected by the firms, and the recruitment of key clinicians for post-marketing studies.
The dominance of DTMs in HTA/CEA can be characterised as a consequence of pharmaco-economic research addressing the market’s failure to summarise the complex information about the health and cost consequences of adopting a new drug at a given drug offer price. DTMs can accommodate and analyse uncertainty in parameters and the impact of uncertainty on optimal decision-making. Therefore, DTMs are the model of choice to solve for the IPER of a new drug, which can then be used in the reimbursement process. However, information about the IPER of a new drug at a given price is not the only information the market fails to provide. DTMs cannot correct for the failure of the market to reveal an IPER generated by the firm that reflects competition in the market for health inputs. This market failure is a consequence of the market power of the seller that arises from patents and the market power of a monopsonist purchaser. For an economic model to be used to analyse this aspect of the real world, it needs to incorporate strategic behaviour.
In summary, molecules do not act strategically, therefore it is appropriate to use DTMs to analyse the consequences (costs and effects) of new drug adoption. They can be used to estimate the IPER of a new drug at a given price and accommodate the associated uncertainty. However, DTMs cannot be used to analyse situations in which people, firms and institutions act strategically when they buy, sell, prescribe and consume these molecules. Therefore DTMs can be used to inform, but not analyse, the decision to reimburse the drug. The Game described in the following section illustrates how strategic behaviour by players with market power can be accommodated in an economic model.
8.4 The New Drug Reimbursement Game
The drug reimbursement game presented in this chapter is far less ambitious than the three published games described above; its aim is to demonstrate new drug price as an equilibrium outcome of reimbursement rather than an exogenous choice by the Firm. It was developed using Grüne-Yanoff and Schweinzer’s (GY-S) Architecture of Game Theory (Grüne-Yanoff and Schweinzer 2008). This architecture characterises game theory as having three components: World, the Model and Theory Proper.
“World” characterises the economic situation which, in an applied economic model, is the justification for the analysis. “Model” comprises a Narrative and a Game Structure. The Narrative is the story that sets out the players, the ordering of events and the justifications for their payoffs. It also clarifies the opportunities for the players to act strategically. The Game Structure is analogous to the decision tree in a DTM. (For example compare the decision tree presented in Fig. 8.1 with the extensive form game presented in Fig. 8.2 in Sect. 8.6.2.1) The Game Structure also includes the formal expression of all the payoffs, conditions, assumptions and parameters. Theory Proper is the theoretical foundation of the problem. Solution Concepts can be thought of as theory expressed as a rule that is used to predict how a player or game will be played. This is most commonly an equilibrium concept, the most well-known of which is the Nash Equilibrium. (See Watson 2002)
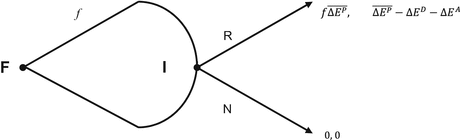

Fig. 8.2
The new drug reimbursement game
The Game’s Narrative, which precedes the Game Structure in a formal expression of the overall problem, is a detailed qualitative description of the Game. The Narrative’s role in the GTM can be thought of as analogous to the body of empirical evidence and associated narratives that support the pharmaco-economic model, as distinct from the technical assumptions in the model.
Grüne-Yanoff and Schweinzer describe a given economic situation as having multiple interpretations and hence solutions.11 The authors describe the role of the Narrative in supporting a game’s solution by directing which of the many possible solution concepts should be applied, hence selecting a concept of rationality from the many possible rather than defining a unique rationality.
Accordingly, the Game presented in this chapter is designed to have sufficient detail in the Narrative to support the Solution Concepts used in the Game, but other concepts.
8.5 World (The Economic Problem)
The Reimburser is about to apply β c as the threshold IPER where:
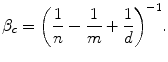
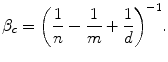
The use of a health shadow price that accommodates the characteristics of the health care sector appeals to the Reimburser. She recognises that allocative inefficiency is a significant feature of health care budgets throughout the OECD (Garber and Skinner 2008). The Reimburser also recognises that there is no Institution analogous to reimbursing institutions that make systematic improvements to allocative efficiency by reallocating funds across existing programmes and technologies (Culyer et al. 2007; Elshaug et al. 2007; Pearson and Littlejohns 2007). She also recognises that displacement could be suboptimal (d < m) and that suboptimality of displacement is not a parameter she can control.
Then the Reimburser thinks about this threshold IPER from the perspective of the Firm. At $5,042 per QALY, β c is significantly lower than the threshold of $75,000 per QALY she used historically. She wonders whether the clinicians are correct: will this lower maximum price mean that drugs that would otherwise have been reimbursed at prices above $5,042 per QALY will no longer be reimbursed? The Reimburser considers the idea of the opportunity cost of these additional high cost drugs. She wonders: if fewer new drugs are available as a consequence, will the population necessarily be worse off than it would have been under the existing maximum IPER of $75,000 per QALY?
The Reimburser asks her Health Economic Adviser how the Institution should respond to the following threat:
If the threshold is reduced from $75,000 to β c = $5,024 per QALY, less than 15 % of the new drugs that would otherwise be approved will be made available to patients. The population will be worse off at the lower threshold IPER.
8.6 Model
The model comprises the Narrative and the Game Structure.
8.6.1 Narrative
The Narrative comprises the Firm’s decision, the Institution’s decision and the rules of new drug reimbursement.
8.6.1.1 The Firm’s Decision
A pharmaceutical firm (the Firm) completes the R&D cycle for a hypothetical new drug for rheumatoid arthritis called Araamax and now two regulatory hurdles need to be cleared. The first hurdle is regulatory approval for clinicians to prescribe Araamax for certain groups of patients. The evidence required for this hurdle is that of the comparative clinical effectiveness of the drug. Specifically, it needs to be demonstrated in a clinical trial that the new drug is no worse than the best existing drug for that condition. We assume that the patent holders have demonstrated that Araamax has superiority (an additional health gain for target patients) against the existing drug (Rathmab) in an appropriate clinical trial; it is clinically innovative. Furthermore, the group of patients for whom Araamax represents a clinical benefit (the target patients) all have the same incremental benefit compared with the best existing therapy and no patients outside this group experience a benefit from this drug.
The second hurdle is approval for government reimbursement of the costs of Araamax for a patient for whom it is prescribed. This approval is for reimbursing the entire cost of Araamax for all patients for whom prescribing is approved (the target group). The evidence required to clear the reimbursement hurdle is that of the price of the new drug expressed as the additional financial cost to the health sector per additional unit of health effect, or in PEA terminology, the IPER. The estimate of this IPER (the evidence) is produced by the Firm and is public information in the context of the Game; it is known to both the Institution and the Firm.
Araamax is patented and, as a monopolist, the Firm needs to select rather than accept the price at which it offers the new drug to the reimbursement authority. We assume it selects the price so as to maximise economic profit.
There are two factors that the Firm needs to take into consideration when selecting an offer price: the marginal cost of production of the drug (which defines the minimum price at which the Firm will be willing to produce the drug) and the Institution’s signal of the threshold IPER; the maximum price the Institution is willing to pay for a new health effect. Each of these are discussed in detail in the following sections.
The Marginal Cost of Production of Araamax
Tables 8.1 to 8.6 set out a “worked example” of the relationships between clinical innovation, price, costs of manufacturing and economic rents. (Corresponding numbers are presented in the text in italics, bracketed and referenced to the tables. These numbers are not used in the model, which is solved algebraically. These numbers are simply illustrative of cost concepts.)
Table 8.1
QALYs per course per patient
Comparison | Incremental QALYs per patient |
|---|---|
Rathmab vs. placebo | 0.05 |
Araamax vs. Rathmab | 0.07 |
Araamax vs. placebo | 0.12a |
Table 8.2
IPER (incremental price per incremental QALY) per course
Comparison | IPER ($) |
|---|---|
Rathmab vs. placebo | 5,000 |
Araamax vs. Rathmab | 75,000 |
Araamax vs. Placebo | 45,833a |
Table 8.3
Expenditure per course
Comparison | Cost of a course ($)a |
|---|---|
Additional cost of a course of Rathmab vs. placebo = cost of a course of Rathmab | 250 |
Additional cost of a course of Araamax compared to Rathmab | 5,250 |
Additional cost of a course of Rathmab vs. placebo = cost of a course of Araamax | 5,500 |
Table 8.4
Cost of manufacture per course
Cost of a course ($) | |
|---|---|
Rathmab | 250(a)a |
Araamax | 250(a)b |
Table 8.5
Per course summary measures
Per course summary measures | Rathmab | Araamax |
|---|---|---|
QALY | 0.05 | 0.12 |
Price | 250 | 5,500 |
Cost of manufacture | 250 | 250 |
Economic rent | 0 | 5,250 |
Table 8.6
Per incremental QALY summary measures
Per incremental QALY measures | Description | Rathmab vs. placebo | Araamax vs. Rathmab |
|---|---|---|---|
IPER | Incremental price per incremental QALY | 5,000a | 75,000 (b) |
IMER | Incremental cost of manufacture per incremental QALY | 5,000b | 0 (b) |
IπERc | Incremental economic rent per incremental QALY = IPER—IMER in this case | 0 | 75,000 (b) |
In this example, incremental measures are compared with their own next best alternative therapy (Rathmab to placebo; and Araamax to Rathmab) and Araamax is also compared to placebo. Three firm related variables are illustrated.
The IPER is the conventional ICER for the drugs, but is referred to as an IPER to capture the endogeneity of price.
The incremental manufacturing cost-effectiveness ratio (IMER) is a ratio of the additional costs of manufacturing the new drug to the additional QALYs of the new drug compared with the next best alternative. A new drug could have clinical innovation, but the costs of producing the new drug are the same as the cost of producing the older drug and hence the IMER could be zero.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


