INTRODUCTION
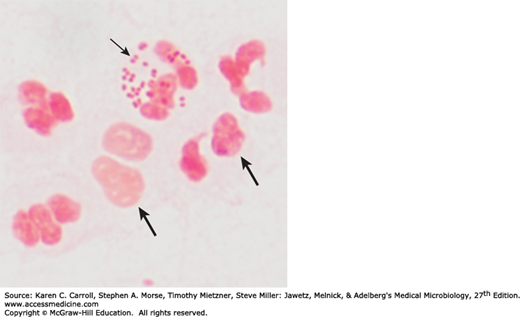
The family Neisseriaceae includes the genera Neisseria, Kingella, Eikenella, Simonsiella, and Alysiella (see Chapter 16). The neisseriae are gram-negative cocci that usually occur in pairs (diplococci). Neisseria gonorrhoeae (gonococci) and Neisseria meningitidis (meningococci) are pathogenic for humans and typically are found associated with or inside polymorphonuclear cells. Some neisseriae are normal inhabitants of the human respiratory tract, rarely if ever cause disease, and occur extracellularly. Members of the group are listed in Table 20-1.
| Acid Formed From | ||||||
|---|---|---|---|---|---|---|
| Growth on MTM, ML, or NYC Mediuma | Glucose | Maltose | Lactose | Sucrose or Fructose | DNAse | |
| Neisseria gonorrhoeae | + | + | − | − | − | − |
| Neisseria meningitidis | + | + | + | − | − | − |
| Neisseria lactamica | + | + | + | + | − | − |
| Neisseria sicca | − | + | + | − | + | − |
| Neisseria subflava | − | + | + | − | ± | − |
| Neisseria mucosa | − | + | + | − | + | − |
| Neisseria flavescens | − | − | − | − | − | − |
| Neisseria cinerea | ± | − | − | − | − | − |
| Neisseria polysaccharea | ± | + | + | − | − | − |
| Neisseria elongata | − | −/w | − | − | − | − |
| Moraxella catarrhalis | − | − | − | − | − | + |
Gonococci and meningococci are closely related, with 70% DNA homology, and are differentiated by a few laboratory tests and specific characteristics. Meningococci have polysaccharide capsules but gonococci do not, and meningococci rarely have plasmids but most gonococci do. Most importantly, the two species are differentiated by the usual clinical presentations of the diseases they cause: Meningococci typically are found in the upper respiratory tract and cause meningitis, but gonococci cause genital infections. However, the clinical spectra of the diseases caused by gonococci and meningococci do overlap.
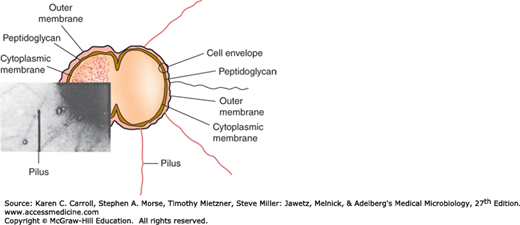
The typical Neisseria is a gram-negative, nonmotile diplococcus, approximately 0.8 μm in diameter (Figures 20-1 and 20-2). Individual cocci are kidney bean shaped; when the organisms occur in pairs, the flat or concave sides are adjacent.
In 48 hours on enriched media (eg, modified Thayer-Martin, Martin-Lewis, GC-Lect, and New York City), gonococci and meningococci form convex, glistening, elevated, mucoid colonies 1–5 mm in diameter. Colonies are transparent or opaque, nonpigmented, and nonhemolytic. Neisseria flavescens, Neisseria cinerea, Neisseria subflava, and Neisseria lactamica may have yellow pigmentation. Neisseria sicca produces opaque, brittle, wrinkled colonies. Moraxella catarrhalis produces nonpigmented or pinkish gray opaque colonies.
The neisseriae grow best under aerobic conditions, but some grow in an anaerobic environment. They have complex growth requirements. Most neisseriae oxidize carbohydrates, producing acid but not gas, and their carbohydrate patterns are a means of distinguishing them (see Table 20-1). The neisseriae produce oxidase and give positive oxidase reactions; the oxidase test is a key test for identifying them. When bacteria are spotted on a filter paper soaked with tetramethylparaphenylenediamine hydrochloride (oxidase), the neisseriae rapidly turn dark purple.
Meningococci and gonococci grow best on media containing complex organic substances such as heated blood, hemin, and animal proteins and in an atmosphere containing 5% CO2 (eg, candle jar). Growth is inhibited by some toxic constituents of the medium (eg, fatty acids or salts). The organisms are rapidly killed by drying, sunlight, moist heat, and many disinfectants. They produce autolytic enzymes that result in rapid swelling and lysis in vitro at 25°C and at an alkaline pH.
NEISSERIA GONORRHOEAE
Gonococci oxidize only glucose and differ antigenically from the other neisseriae. Gonococci usually produce smaller colonies than those of the other neisseriae. Gonococci that require arginine, hypoxanthine, and uracil (Arg−, Hyx−, and Ura− auxotype) tend to grow most slowly on primary culture. Gonococci isolated from clinical specimens or maintained by selective subculture have typical small colonies containing piliated bacteria. On nonselective subculture, larger colonies containing nonpiliated gonococci are also formed. Opaque and transparent variants of both the small and large colony types also occur; the opaque colonies are associated with the presence of a surface-exposed protein, Opa.
N gonorrhoeae is antigenically heterogeneous and capable of changing its surface structures in vitro—and presumably in vivo—to avoid host defenses. Surface structures include the following.
Pili are the hairlike appendages that extend up to several micrometers from the gonococcal surface. They enhance attachment to host cells and resistance to phagocytosis. They are made up of stacked pilin proteins (molecular weight [MW], 17–21 kDa). The amino terminal of the pilin molecule, which contains a high percentage of hydrophobic amino acids, is conserved. The amino acid sequence near the midportion of the molecule also is conserved; this portion of the molecule serves in attachment to host cells and is less prominent in the immune response. The amino acid sequence near the carboxyl terminal is highly variable; this portion of the molecule is most prominent in the immune response. The pilins of almost all strains of N gonorrhoeae are antigenically different, and a single strain can make many antigenically distinct forms of pilin.
Por protein extends through the gonococcal cell membrane. It forms pores in the surface through which some nutrients enter the cell. Por proteins may impact intracellular killing of gonococci within neutrophils by preventing phagosome–lysosome fusion. In addition, variable resistance of gonococci to killing by normal human serum depends on whether Por protein selectively binds to complement components C3b and C4b. The MW of Por varies from 32 to 36 kDa. Each strain of gonococcus expresses only one of two types of Por, but the Por of different strains is antigenically different. Serologic typing of Por by agglutination reactions with monoclonal antibodies was a useful method for studying the epidemiology of N gonorrhoeae. However, this method has been replaced by genotypic methods such as pulsed-field gel electrophoresis, Opa typing, and DNA sequencing.
These proteins function in adhesion of gonococci within colonies and in attachment of gonococci to host cell receptors such as heparin-related compounds and CD66 or carcinoembryonic antigen–related cell adhesion molecules. One portion of the Opa molecule is in the gonococcal outer membrane, and the rest is exposed on the surface. The MW of Opa ranges from 20 to 28 kDa. A strain of gonococcus can express no, one, two, or occasionally three types of Opa, but each strain has 11–12 genes for different Opas. PCR of the opa genes followed by restriction endonuclease digestion, and analysis of subsequent fragments by gel electrophoresis is a useful method of strain typing performed by reference laboratories.
This protein (MW, 30–31 kDa) is antigenically conserved in all gonococci. It is a reduction-modifiable protein (Rmp) and changes its apparent MW when in a reduced state. It associates with Por in the formation of pores in the cell surface.
In contrast to the enteric gram-negative rods (see Chapters 2 and 15), gonococcal lipopolysaccharide (LPS) does not have long O-antigen side chains and is called a lipooligosaccharide (LOS). Its MW is 3–7 kDa. Gonococci can express more than one antigenically different LOS chain simultaneously. Toxicity in gonococcal infections is largely attributable to the endotoxic effects of LOS. Specifically, in the fallopian tube explant model, LOS causes ciliary loss and mucosal cell death.
In a form of molecular mimicry, gonococci make LOS molecules that structurally resemble human cell membrane glycosphingolipids. A structure is depicted in Figure 20-3. The gonococcal LOS and the human glycosphingolipid of the same structural class react with the same monoclonal antibody, indicating the molecular mimicry. The presence on the gonococcal surface of the same surface structures as human cells helps gonococci evade immune recognition.
FIGURE 20-3
Structure of gonococcal lipooligosaccharide, which has lacto-N-neotetraose and a terminal galactosamine in a structure similar to the human ganglioside glycosphingolipid series. The basal oligosaccharide is in light red, and the lacto-N-neotetraose is in dark red. (Courtesy of JM Griffiss.)
The terminal galactose of human glycosphingolipids is often conjugated with sialic acid. Sialic acid is a nine-carbon, 5-N-acetylated ketulosonic acid also called N-acetylneuraminic acid (NANA). Gonococci do not make sialic acid but do make a sialyltransferase that functions to take NANA from the human nucleotide sugar cytidine 5′-monophospho-N-acetylneuraminic acid (CMPNANA) and place the NANA on the terminal galactose of a gonococcal acceptor LOS. This sialylation affects the pathogenesis of gonococcal infection. It makes the gonococci resistant to killing by the human antibody–complement system and interferes with gonococcal binding to receptors on phagocytic cells.
N meningitidis and Haemophilus influenzae make many but not all of the same LOS structures as N gonorrhoeae. The biology of the LOS for the three species and for some of the nonpathogenic Neisseria species is similar. Four of the various serogroups of N meningitidis make different sialic acid capsules (see later discussion), indicating that they also have biosynthetic pathways different from those of gonococci. These four serogroups sialylate their LOS using sialic acid from their endogenous pools.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree