The Musculoskeletal System
Skeletal muscles and bones support and move the body and protect soft tissues and the internal organs. Bones serve as a calcium reservoir and some contain hematopoietic connective tissue that is vital to the formation of blood cells. Muscles are responsible for vascular tone, gut contractions, genitourinary function, and the beating of the heart. Life is impossible if the cardiac or respiratory muscles are destroyed. Some muscles function relatively independently of neural or hormonal stimulation, whereas other muscles are active only in response to neural stimulation. Bones and muscles are joined at the joints by tendons and ligaments. Diseases or injuries to the muscles and bones make movements difficult or painful and have the potential to result in long-term disability.
● Physiologic Concepts
There are three types of muscles: skeletal, cardiac, and smooth. The basic processes of contraction are similar in all three types, but important differences exist. Although the focus of this chapter is the skeletal-muscular system, the unique characteristics of the cardiac and smooth muscles will be presented briefly.
SKELETAL MUSCLE
Skeletal muscles are connected to bones through tendons. Tendons move the bones by contraction of the skeletal muscles, which is controlled by lower
motor neurons from the spinal cord. One motor neuron may innervate several muscle fibers. A motor neuron and all the muscle fibers it innervates are called a motor unit. In general, the muscles over which we have fine control have only a few muscle fibers innervated by a single motor neuron. Muscles that do not need fine control (i.e., the support muscles of the back) are composed of many muscle fibers per motor neuron.
motor neurons from the spinal cord. One motor neuron may innervate several muscle fibers. A motor neuron and all the muscle fibers it innervates are called a motor unit. In general, the muscles over which we have fine control have only a few muscle fibers innervated by a single motor neuron. Muscles that do not need fine control (i.e., the support muscles of the back) are composed of many muscle fibers per motor neuron.
Skeletal muscle cells are highly differentiated cells whose growth during embryogenesis and later in life is under the control of growth factors, hormones, and physical stimuli. During embryogenesis, skeletal muscle cells undergo both hyperplasia (increase in cell number) and hypertrophy (increase in cell size). After embryogenesis, skeletal muscle cells continue to undergo hypertrophy in response to certain stimuli, including exercise, but no longer undergo hyperplasia. The protein myostatin, also known as “growth and differentiating factor-8,” has been identified as playing a key role in the regulation of skeletal muscle growth before and after birth, by limiting the growth and reproduction of muscle cell fibers. In animals that lack the gene coding for myostatin, muscle hypertrophy and hyperplasia occur both before and after birth, resulting in increased muscle mass and strength.
Skeletal Muscle Structure
Each skeletal muscle is made up of many muscle cells, called muscle fibers. A given muscle may have a few hundred or several thousand fibers. The more muscle fibers present in a muscle, the greater the potential strength of that muscle.
Skeletal muscle is called striated muscle because of the banding that can be seen throughout the muscle with a light microscope. The striations reflect the subunits of each muscle fiber: the myofibrils. A single muscle cell is made up of many myofibrils. The myofibrils are composed of smaller subunits called myofilaments; myofilaments are the functional units of the muscle cell. They are composed of thick and thin contractile proteins, grouped together into a repeating pattern, called a sarcomere.
The Sarcomere
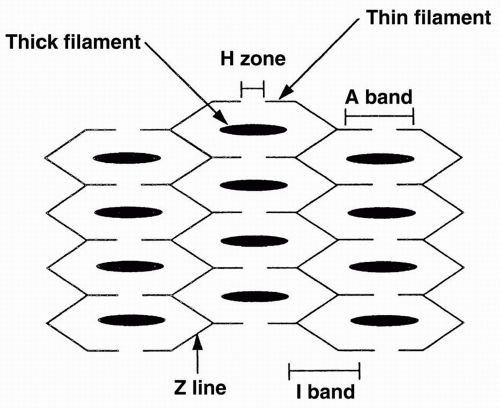
Three sarcomeres are aligned together as shown in Figure 10-1. Each sarcomere contains thick and thin filaments. The thick filaments are located in the central region of the sarcomere, and are composed of several hundred copies of the contractile protein myosin. The thin filaments are attached to the edges of the sarcomere and are composed of the proteins actin, tropomyosin, and troponin.
The area of the sarcomere where only thick filaments are present is called the H zone. The area where only thin filaments are present is termed the I zone. The A band is the section where the thin and thick filaments overlap. The Z lines are the borders of the sarcomere, where the actins attach. Each sarcomere spans from one Z line to the next.
Cross-Bridges
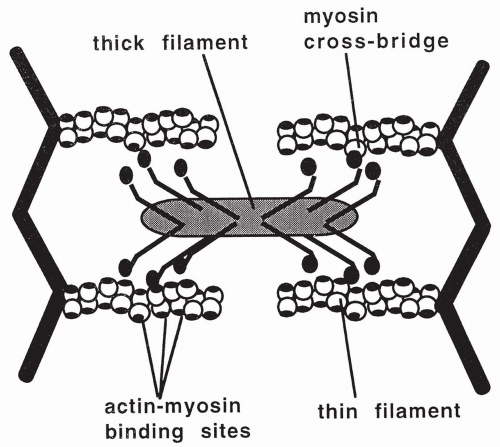
Each myosin molecule is composed of six peptide chains: two heavy chains that twist together to form a long tail with two globular heads, and four light chains that group, two to a head, with the myosin heads. The heads form small projections that extend from the myosin filament. These projections are called cross-bridges. When a muscle is relaxed, the myosin cross-bridges are unattached in the sarcomere. During this relaxed state, an adenosine triphosphate (ATP) molecule binds to myosin and is split into ADP and a high-energy phosphate (P). The ADP and P remain bound to the myosin, without releasing the energy generated by the splitting of ATP.
Muscle Contraction
Contraction of a muscle occurs when the myosin cross-bridges bind to specific sites on the actin proteins. When this occurs, energy that has been stored in the myosin head from the previous splitting of an ATP molecule is released. The released energy is used to swing the cross-bridges, causing the actin and myosin filaments to slide over each other. This shortens and contracts the muscle. With cross-bridge swinging, the remaining ADP and P release from myosin.
During muscle contraction, the lengths of the actin and myosin filaments do not change, but the I band and the H zone shorten. The different regions of the sarcomere are described in Table 10-1; the thick filaments, myosin heads, and thin filaments are shown in Figure 10-2. Each muscle contraction involves several repeated cycles of filament sliding to provide the tension necessary for the muscle to do work.
TABLE 10-1 Sarcomere Composition and Changes During Contraction | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Excitation-Contraction Coupling
Because skeletal muscle contraction only occurs in response to neural stimulation and the subsequent release of intracellular calcium, myosin, and actin cannot always bind to each other so the cross-bridges cannot always swing. This prevents constant muscle contraction.
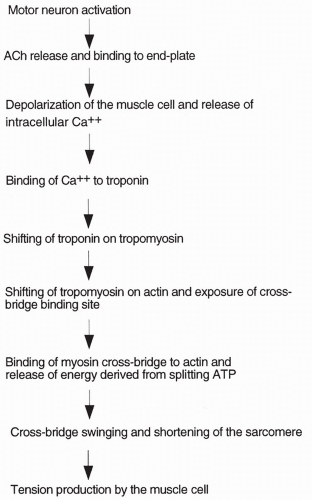
The process of muscle contraction is summarized below. When an action potential is delivered by a motor neuron to a skeletal muscle fiber, the neuron releases acetylcholine (ACh) into the neuromuscular junction. ACh diffuses to
a specialized area of the muscle cell, called the end plate. Muscle cell end plates are concentrated with receptors for ACh. ACh binds to the receptors causing the opening of sodium channels present in the muscle cell. With the opening of these channels, sodium ions rush into the cell, depolarizing (making the cell positively charged on the inside) and initiating an action potential. The action potential passes along the entire muscle fiber, depolarizing the fiber. Depolarization spreads into the fiber through small tubules, called transverse (T) tubules, which run along the juncture between the A and I bands. When the inside of the cell becomes positive, calcium ion is released from intracellular bags of calcium (called lateral sacs) that lie adjacent to the T tubules. The lateral sacs are outpouchings of a large intracellular calcium storage compartment: the sarcoplasmic reticulum causes high levels of intracellular calcium released from the sarcoplasmic reticulum to initiate muscle contraction.
a specialized area of the muscle cell, called the end plate. Muscle cell end plates are concentrated with receptors for ACh. ACh binds to the receptors causing the opening of sodium channels present in the muscle cell. With the opening of these channels, sodium ions rush into the cell, depolarizing (making the cell positively charged on the inside) and initiating an action potential. The action potential passes along the entire muscle fiber, depolarizing the fiber. Depolarization spreads into the fiber through small tubules, called transverse (T) tubules, which run along the juncture between the A and I bands. When the inside of the cell becomes positive, calcium ion is released from intracellular bags of calcium (called lateral sacs) that lie adjacent to the T tubules. The lateral sacs are outpouchings of a large intracellular calcium storage compartment: the sarcoplasmic reticulum causes high levels of intracellular calcium released from the sarcoplasmic reticulum to initiate muscle contraction.
The Role of Intracellular Calcium in Initiating Muscle Contraction
When a skeletal muscle fiber is at rest, the myosin heads are prevented from binding to the actin molecules by the presence of the other two proteins of the thin filaments: tropomyosin and troponin. Without myosin binding to actin, energy from ATP cannot be released, cross-bridges cannot swing, and the muscle cannot contract. Elevated intracellular calcium changes the interaction of these proteins and causes contraction.
At rest, tropomyosin is attached to the actin molecules in such a way that it blocks the sites on actin where the myosin cross-bridges would bind. Troponin attaches to both the actin and the tropomyosin molecules. It also has a binding site for calcium. When calcium concentration inside the cell increases, calcium binds to troponin, causing troponin to shift its position on the tropomyosin molecule. This causes tropomyosin to shift its position on actin, uncovering the binding site for myosin. Once the binding site on actin is uncovered, the myosin heads immediately bind actin and release their stored energy, and the cross-bridges swing. The filaments slide past each other and the muscle contracts. The greater the number of cross-bridges connected and swinging at one time, the greater the tension produced by the muscle. Excitation-contraction coupling and the role of calcium are outlined in Figure 10-3.
After each cross-bridge swinging, a new ATP molecule binds to the myosin molecule (the old ADP and P have already been released). This causes the myosin cross-bridges to separate from actin and the fiber to relax. Once relaxed, the new ATP molecule is split, and its energy is again stored in the myosin head. If calcium is still high intracellularly, the myosin cross-bridge will again bind actin, and this energy will be released, leading to a second contraction. Excitation-contraction coupling occurs when intracellular calcium levels increase from a resting molar concentration of less than 10−7 to approximately 10−5. During a typical action potential, calcium concentration is approximately 2 × 10−4 molar; this is approximately 10 times the level required to maximally contract the muscle.
Muscle Fiber Summation
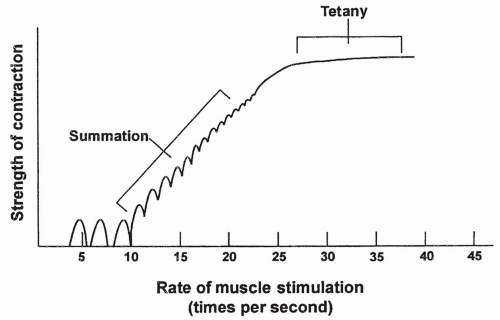
Each calcium pulse lasts approximately 1/20th of a second and produces what is called a single muscle twitch. Summation occurs if calcium is maintained in the intracellular compartment by repeated neural stimulation of the muscle. Summation means individual twitches are added together, causing increased contraction strength. If stimulation is prolonged, the individual twitches blend together until the strength of contraction is at a maximum. At this point, the muscle is said to have reached tetany, which is characterized by a smooth, continued contraction. Summation and tetany in an individual muscle fiber are shown in Figure 10-4.
Whole-Muscle Summation: Multiple-Fiber Summation
The total amount of tension produced by an entire muscle is the result of the summation of the tension produced by each muscle fiber. An increase in the number of fibers stimulated to contract will increase the amount of tension produced by the entire muscle. This is called multiple-fiber summation. Multiple-fiber summation occurs when additional motor units are activated, leading to the contraction of more muscle fibers.
Relaxation of the Muscle
Muscle fibers relax when calcium is pumped out of the cytoplasm back into the sarcoplasmic reticulum. Calcium pumping is an active process occurring in the membrane of the sarcoplasmic reticulum. This process uses energy derived from splitting a different ATP molecule. When calcium levels decrease to approximately 10−7 molar, troponin returns to its original position on the tropomyosin molecule, and tropomyosin again inhibits the binding of actin and myosin, which causes muscle contraction to stop.
Muscle Metabolism and Muscle Fatigue
Muscle contraction depends on the production of ATP from one of three sources: (1) creatinine phosphate (CP) stored in the muscle, (2) oxidative phosphorylation of foodstuffs stored in or delivered to the muscle, and (3) anaerobic glycolysis. Muscle fatigue results when the use of ATP in a muscle becomes excessive.
When a muscle first starts contracting, it begins to use its stores of CP to drive contraction. CP contains a high-energy phosphate molecule that it transfers to ADP to produce ATP: CP + ADP = C + ATP.
This source of ATP is rapidly accessed, but is limited by the amount of CP present in the cell at the start of contraction. After several seconds, the muscle begins to rely mostly on oxidative phosphorylation. Sources of fuel for oxidative phosphorylation include glycogen stored in the muscle and, later, glucose and fatty acids delivered to the muscle in the blood supply. This source of energy is available for 30 minutes or so, depending on the intensity of contraction. If the intensity of exercise is very high, or the duration is very long, the muscle begins to rely increasingly on anaerobic glycolysis. Anaerobic glycolysis produces a limited amount of ATP from the metabolism of muscle glycogen and circulating blood glucose. A muscle using anaerobic glycolysis for a large part of its ATP production rapidly fatigues. Muscle fatigue can be predicted experimentally by depletion of glycogen stored in the muscle. Lactic acid is a by-product of anaerobic glycolysis and may accumulate in the muscle and blood with intense or prolonged muscle contraction, contributing to fatigue. Lactic acid may also contribute to the muscle pain felt a day or two after intense exercise.
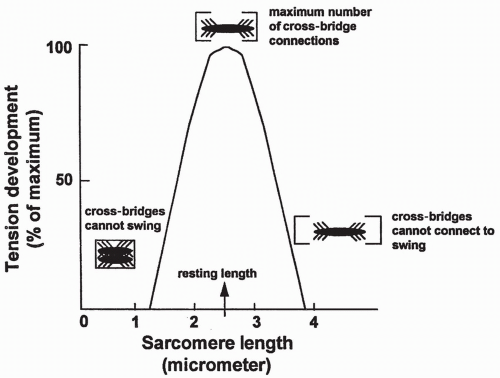
Length-Tension Relationship
The resting length of a muscle fiber determines the maximum amount of tension it can produce. This relationship is shown in Figure 10-5. Muscle fiber length affects tension production as a result of stretching of the sarcomere. If
a sarcomere is stretched beyond an optimum length, as shown on the right side of the figure, some myosin cross-bridges will be too far away to connect with the actin sites and therefore will not swing. This will reduce total tension. In contrast, if the sarcomere is less than optimally stretched, as shown on the left side of the figure, the cross-bridges will be bunched too closely together to be able to swing freely, thus limiting filament sliding and again reducing total tension. In a normal skeletal muscle, muscle length at rest will produce the maximum amount of tension.
a sarcomere is stretched beyond an optimum length, as shown on the right side of the figure, some myosin cross-bridges will be too far away to connect with the actin sites and therefore will not swing. This will reduce total tension. In contrast, if the sarcomere is less than optimally stretched, as shown on the left side of the figure, the cross-bridges will be bunched too closely together to be able to swing freely, thus limiting filament sliding and again reducing total tension. In a normal skeletal muscle, muscle length at rest will produce the maximum amount of tension.
Isometric Contraction
Isometric contractions are those in which cross-bridge swinging occurs and tension is produced without shortening of the muscle. An isometric contraction occurs when an individual is trying to lift a load that requires greater tension than can be produced by the muscle. No mechanical work is performed. Tension is produced, but the muscle does not change in length.
Isotonic Contraction
Isotonic contractions occur when a muscle shortens against a constant load. Work is done to lift the load. An example of an isotonic contraction is when a weightlifter lifts a barbell. The tension remains the same, but the muscle length changes. Most muscle contractions include both isotonic and isometric periods.
Series Elastic Elements
There is typically a delay between excitation of a muscle and an isotonic contraction. A delay occurs because the elastic components of the muscle, including the tendons and the attachments of the sarcomeres, must be shortened before the muscle itself shortens. The elastic components of a muscle are called the series elastic elements. If a second contraction of the muscle occurs before the series elastic elements relax, there is no delay, and muscle tension can be increased immediately. This concept of the series elastic element may be easier understood by picturing a spring connected to an object. Before the object can be lifted by pulling the spring, the spring must first be stretched. This delays lifting of the object.
Fast-Twitch and Slow-Twitch Fibers
Different muscles contain different types of muscle fibers, depending on the range of jobs performed. Muscles that must function continually, such as those of the respiratory system, must have long endurance and an ample supply of oxygen. Others function briefly and intensely and then relax; these muscles must be able to produce short bursts of high energy. Usually, a muscle will
contain a mixture of fiber types, with one fiber type predominating but not exclusively. Two main divisions of muscle fibers are fast-twitch and slow-twitch fibers.
contain a mixture of fiber types, with one fiber type predominating but not exclusively. Two main divisions of muscle fibers are fast-twitch and slow-twitch fibers.
Fast-twitch fibers release calcium rapidly from the sarcoplasmic reticulum, and rapidly split ATP to ADP on the myosin head. This causes the rate of cross-bridge swinging to be fast. Fast-twitch fibers may depend primarily on either oxidative phosphorylation or anaerobic glycolysis for energy, depending on what type of work they typically perform.
Fast-twitch fibers that frequently produce large amounts of energy for quick bursts of tension have large stores of glycolytic enzymes and produce a great deal of their ATP from anaerobic glycolysis. These are usually large fibers and require less vascularization because they rely less on oxidative phosphorylation; therefore, they appear white. These fibers are called fast-glycolytic fibers. Fast-glycolytic fibers tire rapidly and predominate in the muscles of weightlifters and sprinters.
Fast-twitch fibers that rely mostly on oxidative phosphorylation, called fast-oxidative fibers, are well vascularized and contain elevated stores of the muscle protein myoglobin. Myoglobin combines in the muscle with oxygen, serving as an oxygen storage bank. Fast-oxidative fibers tire less rapidly and predominate in muscles of longer distance runners.
Slow-twitch fibers are small, highly vascularized fibers that depend predominantly on oxidative phosphorylation for the production of ATP. Muscles with slow-twitch fibers look red because of their high vascularity and the presence of the protein myoglobin. Slow-oxidative fibers have long endurance and predominate in muscles required to produce tension for prolonged periods, such as back muscles.
Stretch Reflex
Many skeletal muscles contain special muscle fibers that act as stretch receptors, called muscle spindle fibers. Muscle spindle fibers are fibers wrapped by afferent nerve endings, which increase their rate of firing when the muscle is stretched. The impulses are transmitted to the spinal cord by an afferent neuron. In the spinal cord, the afferent neuron synapses directly on the motor neuron supplying the muscle (a monosynaptic reflex) or on an interneuron, which then stimulates the motor neuron (a multisynaptic reflex). Activation of the motor neuron causes the muscle to contract, thus removing the stretch on the muscle spindles and returning the nerves’ firing rate to normal. This process is called the stretch reflex. The opposite occurs if stretch on the spindles is suddenly reduced (called the negative stretch reflex). The result of either type of stretch reflex is maintenance of the muscle at a resting length. Voluntary muscle movement involves simultaneous contraction of regular muscle fibers and muscle spindle fibers. This contraction allows movements to be fluid. The afferent neurons that innervate the muscle spindle fibers are called gamma
neurons. The monosynaptic stretch reflex that results in the knee-jerk response is shown in Figure 10-6.
neurons. The monosynaptic stretch reflex that results in the knee-jerk response is shown in Figure 10-6.
CARDIAC MUSCLE
Myocardial cells (myocytes) are long, narrow fibers. Cardiac muscle contraction is similar to skeletal muscle contraction, with the following differences:
Cardiac cells are capable of spontaneous contraction; that is, contraction without neural stimulation. Neural stimulation can increase or decrease the rate of cardiac contraction.
Cardiac muscle fibers are connected to each other through areas of low resistance, called intercalated disks. The plasma membrane of adjacent cardiac fibers connects in this area. Intercalated disks allow depolarization, beginning in one cardiac muscle fiber, to pass rapidly to neighboring fibers, ensuring simultaneous contraction of all cardiac muscle fibers at one time. Simultaneous contraction is required for the maintenance of cardiac output and blood pressure.
Two sources of calcium are involved in producing a cardiac muscle cell contraction. In cardiac muscle, as in skeletal muscle, calcium ions are released intracellularly from the sarcoplasmic reticulum, but they also enter the cell from the extracellular fluid through sodium-calcium channels present in
the T tubules. These channels are also voltage sensitive but are slow to open and thus prolong the duration of the cardiac action potential. The strength of cardiac contraction, therefore, is highly dependent on extracellular calcium level. In contrast, skeletal muscle contraction does not depend on extracellular calcium.
Because of the slow calcium channels, cardiac muscle cell contraction lasts approximately 10 times as long as skeletal muscle contraction. As a result, cardiac muscle is unable to fire action potentials rapidly and does not undergo summation or tetany. If the cardiac muscle were to achieve a state of maintained contraction, the heart would be unable to fill with blood.
At rest, cardiac muscle cells are stretched less than is required to produce maximum tension, which allows the heart to increase tension when it is stretched during times of increased filling (e.g., during exercise).
SMOOTH MUSCLE
Smooth muscle contraction and skeletal muscle contraction have some similarities and some important differences. Smooth muscle contraction is not the same in all smooth muscles. Some characteristics of smooth muscle contraction include the following:
Smooth muscle is innervated and stimulated by the sympathetic and parasympathetic nerves of the autonomic nervous system. These nerves do not innervate the smooth muscle at specific end plates, but branch over the muscle cells and diffusely release transmitter substances onto the fibers.
Some smooth muscles function as a unit composed of millions of fibers. These fibers contract in response to action potentials produced from mechanical stretch, local chemical mediator release, or neural or hormonal stimulation. Spontaneous firing of action potentials can also occur. In this type of smooth muscle, action potentials generated from any source pass from one cell to another across gap junctions. This type of smooth muscle is called single-unit smooth muscle. It is found in the gut, throughout the genitourinary tract, and in many blood vessels.
Some smooth muscle fibers contract individually and only in response to neural stimulation. These fibers are usually innervated by one neuron that releases ACh or norepinephrine. These fibers depolarize and contract, but usually do not fire action potentials. This type of smooth muscle is called multi-unit smooth muscle. It is found in the muscles of the eyes and in the muscles that surround hair follicles. When contracted, these muscles cause the hair to stand up on the skin.
Although smooth muscle contains actin and myosin and splits ATP to produce tension, the thin filaments in smooth muscle fibers do not contain troponin. When intracellular calcium levels increase in smooth muscle fibers,
calcium binds to a protein called calmodulin, resulting in phosphorylation of one of the light chains of the myosin heads. Phosphorylation of the light chain allows the myosin head to bind to actin and split ATP.
The sarcomeres of smooth muscle do not show striations under the microscope, but are more diffuse and less regular in pattern, allowing the muscle to contract over a wide range of lengths. There are many more actin molecules than myosin molecules in smooth muscle fibers, although maximal tension production is similar.
In smooth muscle, most calcium enters from the extracellular fluid through voltage-sensitive calcium channels. Some calcium is released from the sarcoplasmic reticulum. In some smooth muscles, intracellular calcium levels always are sufficient to maintain a low level of cross-bridge connection. This results in a resting muscle tone in these muscles.
The speed of cross-bridge cycling and muscle contraction is reduced in smooth muscle compared to skeletal muscle, most likely because myosin heads contain less ATPase. Therefore, it takes longer for ATP to be split, prolonging the amount of time myosin is attached to actin. A longer period of attachment results in increased production of tension. The slow speed of the calcium pumps in smooth muscle also prolongs contraction.
A latch mechanism in smooth muscle allows muscle contraction to be maintained for long periods of time at a fraction of the energy expenditure of skeletal muscle. This mechanism is probably related to the length of time myosin remains attached to actin.
TENDONS
Tendons are bundles of collagen fibers that anchor the muscles to the bones. Tendons transmit force generated by the contracting muscle to the bone and thereby move the bone. The collagen fibers are considered connective tissue and are produced by fibroblast cells. The dense fibrous connective tissue forming tendons gives them great strength.
LIGAMENTS
Ligaments are cords of strong fibrous connective tissue that join bone to bone, usually at joints. Ligaments grow out of the periosteum and allow and limit joint movement.
BONES
Bone Structure
Mature bone is composed of 30% organic (living) material and 70% salt deposits. The organic material is called the matrix, and is composed of more than
90% collagen fibers and less than 10% proteoglycans (proteins plus polysaccharides). The salt deposits are primarily calcium and phosphate, with small amounts of sodium, potassium carbonate, and magnesium ions. The salts cover the matrix and are bound to the collagen fibers by the proteoglycans. The organic matrix gives bone its tensile strength (resistance to being pulled apart). The bone salts give bone its compressional strength (ability to withstand compression).
90% collagen fibers and less than 10% proteoglycans (proteins plus polysaccharides). The salt deposits are primarily calcium and phosphate, with small amounts of sodium, potassium carbonate, and magnesium ions. The salts cover the matrix and are bound to the collagen fibers by the proteoglycans. The organic matrix gives bone its tensile strength (resistance to being pulled apart). The bone salts give bone its compressional strength (ability to withstand compression).
Exchangeable Calcium
Some calcium ion in bone is noncrystallized. This noncrystalline salt is considered exchangeable calcium, in that it can rapidly move between the bone, interstitial fluid, and blood.
Bone Formation
Bone formation is ongoing and can involve the lengthening and thickening of the bone. The rate of bone formation changes throughout the lifespan. Bone formation is determined by hormonal stimulation, dietary factors, and the amount of stress put on a bone, and results from activities of the bone-forming cells, the osteoblasts.
Osteoblasts are found on the outer surface and on the inside of bones. Osteoblasts respond to various chemical signals to produce the organic matrix. When the organic matrix is first produced, it is called the osteoid. Within a few days, calcium salts begin to precipitate on the osteoid and the bone hardens over the next several weeks or months. Some osteoblasts remain part of the osteoid, and are called osteocytes or true bone cells. As the bone forms, osteocytes in the matrix send out projections to each other, forming a system of microscopic canals (canaliculi) in the bone.
Osteoblast activity is affected by diet, hormonal stimulation, and exercise. These factors interact and are dynamic, resulting in different rates of bone formation throughout a lifetime.
Exercise and Osteoblast Activity
Osteoblastic activity is stimulated by exercise and weight-bearing, as a result of the electrical currents produced when stress is applied to the bone. Bone fracture dramatically stimulates osteoblast activity, but the exact mechanism is unclear.
Hormonal Stimulation and Osteoblast Activity
Estrogen, testosterone, and growth hormone enhance osteoblast activity and bone growth. Bone growth is accelerated during puberty as a result of surging levels of these hormones. Estrogen and testosterone eventually cause the long bones to stop growing by stimulating closure of the epiphyseal plate (growing end of the bone). When estrogen levels decrease after menopause, osteoblastic activity is reduced. Deficiencies in growth hormone impede bone formation.
Diet and Osteoblast Activity
An adequate diet during childhood and adolescence is essential for maximal bone growth. Calcium ion deficiency during adolescence will result in bones that are less dense than optimum later in life. Most of the calcium present in bones in an individual’s lifetime is deposited before the age of 20.
Vitamin D Control of Osteoblast Activity
Vitamin D stimulates bone calcification directly by acting on the osteoblasts, and indirectly by stimulating calcium absorption across the gut. Increased calcium absorption increases blood calcium concentration, which promotes bone calcification. Thus, vitamin D is essential in order to ensure adequate calcium absorption across the gut. Very large amounts of vitamin D, however, may increase bone breakdown in an attempt to liberate calcium in order to increase serum calcium levels. Large amounts of vitamin D without adequate calcium in the diet actually can promote bone resorption.
Bone Breakdown
Bone breakdown, called resorption, occurs simultaneously with bone formation and is also ongoing throughout life. Bone resorption results from the activity of cells called osteoclasts. Osteoclasts are multinucleated, large phagocytic cells derived from monocytes (white blood cells) present in the bone. Osteoclasts secrete various acids and enzymes that digest the bone and allow for its phagocytosis. Osteoclasts also secrete various cytokines that further stimulate resorption. Osteoclasts are usually present in only one small section of bone at a time, and phagocytize the bone section by section. Once they finish in one area, the osteoclasts disappear and osteoblasts arrive. The osteoblasts begin to fill in the clear section with new bone. This process allows old, weakened bone to be replaced with new, stronger bone.
Factors that control osteoclast activity include parathyroid hormone and calcitonin. Parathyroid hormone tends to increase the number and resorptive capacity of osteoclasts while calcitonin reduces the number and resorptive capacity of osteoclasts.
Parathyroid Hormone and Osteoclastic Activity
Osteoclast activity is primarily controlled by parathyroid hormone, which is released by the parathyroid glands located directly behind the thyroid gland. Parathyroid hormone release increases in response to decreased serum calcium levels. Parathyroid hormone increases osteoclastic activity and stimulates bone breakdown, liberating free calcium into the blood. Increased serum calcium acts in a negative feedback manner to reduce further release of parathyroid hormone. It has been hypothesized that estrogen reduces bone resorption by inhibiting the effect of parathyroid hormone on osteoclasts; the mechanism of this is unknown.
Other Effects of Parathyroid Hormone
Parathyroid hormone maintains serum calcium levels by:
Initiating calcium release from the bones.
Simulating tubular reabsorption of calcium.
Enhancing intestinal absorption of calcium through vitamin D activation.
Increasing renal excretion of phosphate ion → decreasing blood phosphate levels.
Calcitonin and Osteoclastic Activity
Calcitonin is a hormone secreted by the thyroid gland in response to high serum calcium. It has a weak effect on inhibiting osteoclastic activity and formation. Calcitonin causes calcium to be sequestered in bone cells and decreases renal tubular reabsorption of calcium and phosphate. These effects increase bone calcification, thereby reducing serum calcium levels.
Remodeling
The balance between osteoblast and osteoclast activity continually remodels, or renews, the bone. In children and teenagers, osteoblastic activity outpaces osteoclastic activity, leading to thickening and lengthening of the skeleton. Osteoblastic activity also outpaces osteoclastic activity in bones healing from fracture. In a young adult, osteoblastic activity and osteoclastic activity are typically in equilibrium, resulting in a constant total amount of bone mass. By middle age, osteoclastic activity outpaces osteoblastic activity and bone density begins to decrease. Osteoclastic activity is also accelerated in immobilized bones. By the seventh or eighth decade of life, dominance of osteoclastic activity may cause the bones to become brittle, leading to increased fractures. Osteoclastic activities are controlled by several physical and hormonal factors.
Types of Bones
Bone is classified as long, short, flat, or irregular. Long bones are found in the extremities, whereas short bones are found in the ankles and wrists. Flat bones are found in the skull and rib cage. Irregular bones include the vertebrae, the bones of the face, and the jaw.
A long bone consist of a long, thick shaft, called the diaphysis, and two ends, called the epiphyses. Proximal to each epiphysis is the metaphysis. In between the epiphysis and the metaphysis is an area of growing cartilage, called the epiphyseal or growth plate. Long bones grow by means of the accumulation of cartilage at the epiphyseal plate. Cartilage is replaced by the osteoblasts, and the bone elongates. By the end of the teen years, the cartilage is used up, the epiphyseal plate fuses, and the bones stop growing. Growth hormone, estrogen, and testosterone stimulate growth of long bones. Estrogen, in conjunction with
testosterone, stimulates fusion of the epiphyseal plates. The shaft of a long bone is hollowed out along the medullary canal, which is filled with bone marrow.
testosterone, stimulates fusion of the epiphyseal plates. The shaft of a long bone is hollowed out along the medullary canal, which is filled with bone marrow.
Bone Marrow
Bone marrow consists of cells involved in blood cell formation (red marrow) and fat cells (yellow marrow). Marrow is found in long and flat irregular bones. Bone marrow biopsy is performed on flat bones.
JOINTS
Joints are areas of the body where two bones come together. A joint may be freely movable, called a diarthrodial joint, or may be immobile, called a synarthrodial joint.
In a diarthrodial joint, the two ends of the bone are not connected directly, but come together in a fibrous joint capsule that surrounds and supports the joint. There are two layers of the joint capsule: an outer layer and an inner membrane layer called the synovium or synovial membrane. The synovial membrane secretes a slippery fluid, called synovial fluid, which lubricates the joint. The synovial membrane also covers the tendons that connect the bone to muscle, and the ligaments that connect the bones to each other. There is a well-developed vascular supply to the synovium, which may be damaged with joint trauma, leading to swelling, bruising, and pain surrounding the joint. In some joints, the synovial membrane forms a closed sac external to the joint, called a bursa. Bursae are found in areas where the bones are physically close together, or where a tendon runs over the bone. Bursae too may become inflamed, a condition called bursitis. Most joints in the body are diarthrodial joints, including the sacroiliac joint, the interphalangeal joints, the hip and knee joints, and the shoulder and elbow joints. Although all diarthrodial joints are considered movable, some of these joints move more than others (i.e., the sacroiliac joint is nearly fixed, whereas the shoulder joint is capable of moving in several different directions).
In synarthroses, the bones are held together by connective tissue, cartilage, ligaments, or other bones; thus, their positions are, to a large degree, fixed. Examples of synarthroses are the joints of the skull bones, ribs, and intervertebral disks.
● Pathophysiologic Concepts
ATROPHY
Atrophy is the decrease in size of a cell or tissue. Muscle atrophy may result from muscle disuse or severing of the nerve supplying the muscle. With muscular atrophy, the size of the myofibrils is reduced. Although bones do not atrophy, bone density can decrease with disuse or metabolic deficiencies or disease.
STRAINS
A strain is trauma to a muscle or tendon, usually occurring when the muscle or tendon is stretched beyond its normal limit. Strains may involve tissue tears or ruptures. Inflammation occurs with injury to muscles or tendons, leading to pain and swelling of tissue. Healing may take several weeks.
SPRAINS
A sprain is trauma to a joint, usually related to a ligament injury. In a severe sprain, the ligament may be completely torn or ruptured. Sprains lead to inflammation, swelling, and pain. Healing may take several weeks.
JOINT DISLOCATION
Dislocation of a joint occurs when a bone is displaced from its position in a joint. A subluxation is a partial dislocation of the joint in which the ends of the bone remain in partial contact with each other. Joint dislocation typically occurs after a severe trauma, which disrupts the ability of the ligament to hold the bone in place. Dislocation of a joint may also occur congenitally; for example, dislocation of the hip is sometimes seen in a newborn (developmental hip dysplasia). Pathologic dislocations may follow long-term problems such as infection, arthritis, paralysis, or neuromuscular disease. For a traumainduced dislocation, there is associated marked pain, swelling, deformity, and loss of range of motion of the joint. Sometimes a popping noise may be heard or felt at the time of occurrence or during physical examination; in the newborn examination, manipulation of the joint to reproduce the sound or feeling of dislocation is used to diagnose the condition. Dislocation of a joint will usually be apparent on a radiograph and is treated by manipulation or surgical repair followed by immobilization until the joint structures are healed.