Chapter 28 The Metabolism of Purines and Pyrimidines
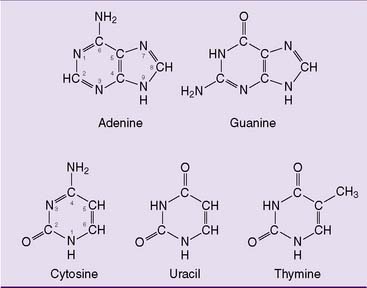
The purine and pyrimidine bases (Fig. 28.1) are constituents of nucleotides and nucleic acids. The ribonucleotides adenosine triphosphate (ATP), guanosine triphosphate (GTP), uridine triphosphate (UTP), and cytidine triphosphate (CTP) are present in millimolar concentrations in the cell. They serve important coenzyme functions in addition to being precursors of RNA synthesis. The deoxyribonucleotides deoxyadenosine triphosphate (dATP), deoxyguanosine triphosphate (dGTP), deoxycytidine triphosphate (dCTP), and deoxythymidine triphosphate (dTTP) are present in micromolar concentrations and are required only for DNA replication and DNA repair. Their cellular concentrations are highest during S phase of the cell cycle.
Purine synthesis starts with ribose-5-phosphate
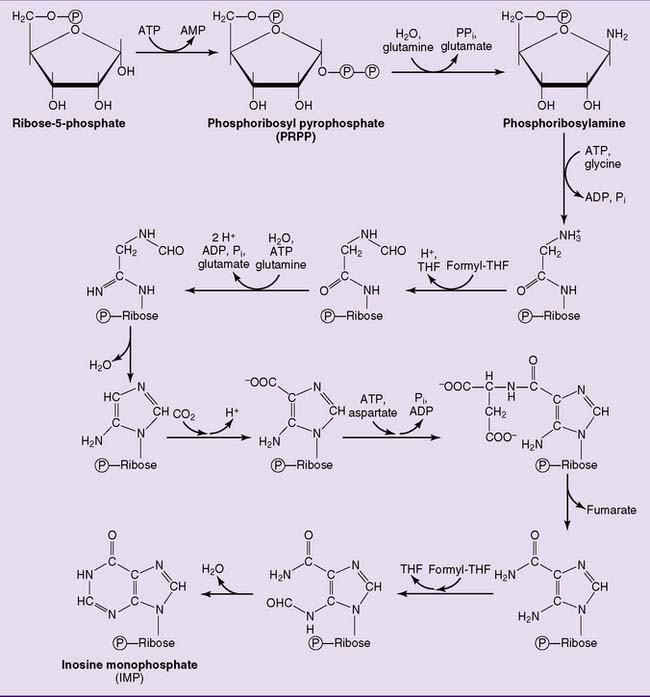
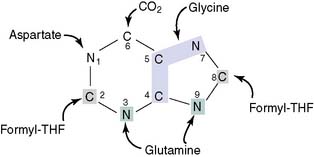
The pathway of purine biosynthesis is shown in Figure 28.2. It starts with ribose-5-phosphate, a product of the pentose phosphate pathway (see Chapter 22). In the reactions of the pathway, all of them cytoplasmic, the purine ring system is built up step by step, with C-1 of ribose-5-phosphate used as a primer.
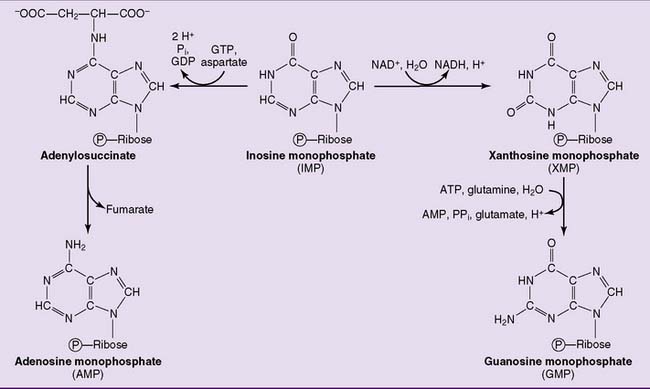
where THF = tetrahydrofolate. The first nucleotide formed in the pathway is inosine monophosphate (IMP), which contains the base hypoxanthine. IMP is a branch point in the synthesis of AMP and GMP (Fig. 28.3). These nucleoside monophosphates are in equilibrium with their corresponding diphosphates and triphosphates through kinase reactions.
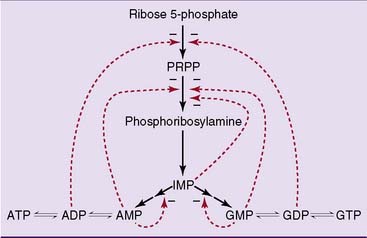
As expected, purine synthesis is regulated by feedback inhibition (Fig. 28.4). The first two enzymes of the pathway, PRPP synthetase and PRPP amidotransferase, are inhibited by the purine nucleotides. In addition, the reactions leading from IMP to AMP and GMP are feedback inhibited by the end products.
Purines are degraded to uric acid
The degradation of purine nucleotides starts with the hydrolytic removal of phosphate from the nucleotides. The nucleosides thus formed are then cleaved into free base and ribose-1-phosphate by purine nucleoside phosphorylase. Adenosine is a poor substrate of the nucleoside phosphorylase. Therefore it is deaminated to inosine first (Fig. 28.5).
Uric acid is the end product of purine degradation in humans. It is synthesized by xanthine oxidase via hypoxanthine and xanthine (reaction  in Figure 28.5). This enzyme contains flavin adenine dinucleotide (FAD), nonheme iron, and molybdenum. Like other nonmitochondrial flavoproteins, it regenerates its FAD by transferring hydrogen from FADH2 to molecular oxygen, forming hydrogen peroxide.
in Figure 28.5). This enzyme contains flavin adenine dinucleotide (FAD), nonheme iron, and molybdenum. Like other nonmitochondrial flavoproteins, it regenerates its FAD by transferring hydrogen from FADH2 to molecular oxygen, forming hydrogen peroxide.
Pyrimidines are synthesized from carbamoyl phosphate and aspartate
Unlike the purine ring, the pyrimidine ring is synthesized before the ribose is added (Fig. 28.6). The pathway starts with carbamoyl phosphate and aspartate, and orotic acid is formed as the first pyrimidine. Orotic acid is processed to the uridine nucleotides, which are the precursors of the cytidine nucleotides. The enzymes of the pathway are cytosolic except for dihydroorotate dehydrogenase (reaction  in Figure 28.6), which is on the outer surface of the inner mitochondrial membrane.
in Figure 28.6), which is on the outer surface of the inner mitochondrial membrane.
The first three enzymes of the pathway, including carbamoyl phosphate synthetase II, aspartate transcarbamoylase, and dihydroorotate dehydrogenase (enzymes 1, 2, and 3 in Fig. 28.6), are formed by different domains of a single large polypeptide. Both this multienzyme complex and the CTP synthetase are feedback inhibited by CTP.
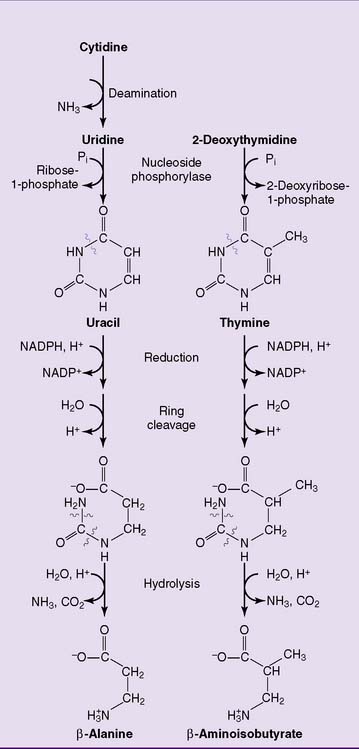
The pyrimidines are degraded to water-soluble products that are either excreted as such or oxidized to carbon dioxide and water (Fig. 28.7).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


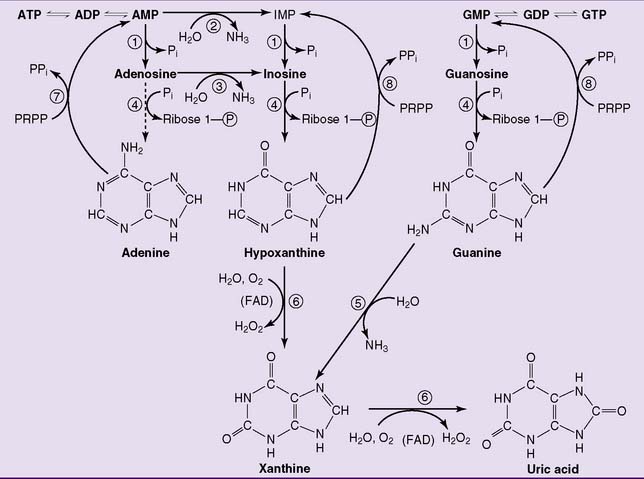
 , 5′-Nucleotidase;
, 5′-Nucleotidase;  , AMP deaminase;
, AMP deaminase;  , adenosine deaminase;
, adenosine deaminase;  , purine nucleoside phosphorylase;
, purine nucleoside phosphorylase;  , guanine deaminase;
, guanine deaminase;  , xanthine oxidase;
, xanthine oxidase;  , adenine phosphoribosyltransferase;
, adenine phosphoribosyltransferase;  , hypoxanthine-guanine phosphoribosyltransferase. IMP, Inosine monophosphate; PRPP, 5-phosphoribosyl-1-pyrophosphate.
, hypoxanthine-guanine phosphoribosyltransferase. IMP, Inosine monophosphate; PRPP, 5-phosphoribosyl-1-pyrophosphate.
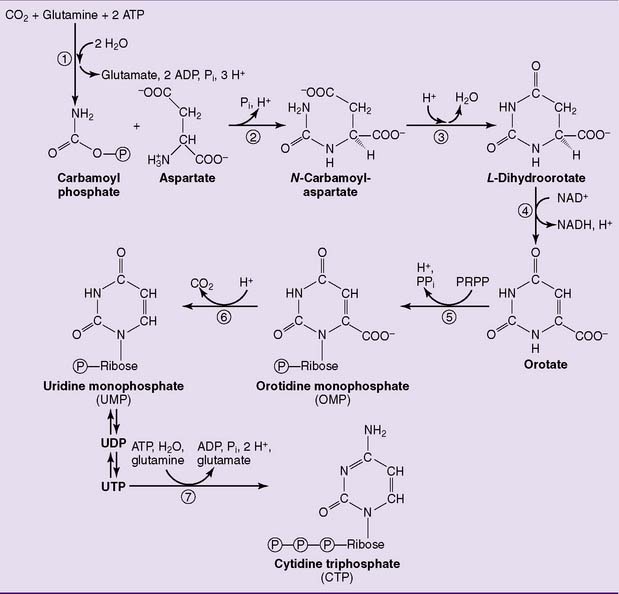
 , Carbamoyl phosphate synthetase II;
, Carbamoyl phosphate synthetase II;  , aspartate transcarbamoylase;
, aspartate transcarbamoylase;  , dihydroorotase;
, dihydroorotase;  , dihydroorotate dehydrogenase;
, dihydroorotate dehydrogenase;  , orotate phosphoribosyltransferase;
, orotate phosphoribosyltransferase;  , orotidylate decarboxylase;
, orotidylate decarboxylase;  , CTP synthetase. PRPP, 5-Phosphoribosyl-1-pyrophosphate.
, CTP synthetase. PRPP, 5-Phosphoribosyl-1-pyrophosphate.