Biological basis of schizophrenia
There is a strong genetic component to schizophrenia with several risk genes that affect early brain development and predispose an individual to developing the condition. Triggers which impact further on neurodevelopment, such as prenatal exposure to viral infections or obstetric complications, probably only lead to the disease in those with a genetic predisposition. Many neurobiological abnormalities have been described in schizophrenia, including disturbances in neuronal numbers and synaptic connections in the cortical, thalamic and hippocampal areas. These disturbances become more marked as the illness progresses, but the heterogenous nature of the disease makes it difficult to determine the precise underlying neuropathology.
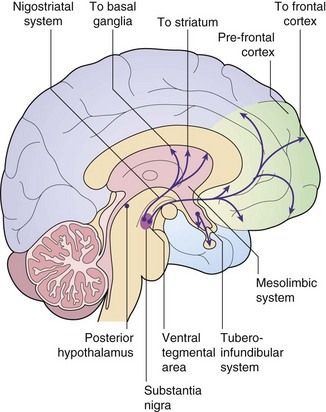
Dopamine–glutamate interactions and their possible involvement in schizophrenia: The limbic region and prefrontal cortex are involved in cognition, emotional memory and the initiation of behaviour. They are regulated by a complex interplay among their neuronal connections, with dopamine and glutamate as important neurotransmitters. Most dopaminergic pathways in the central nervous system (CNS) arise from the substantia nigra (among the basal ganglia) and the ventral tegmental area in the midbrain (Fig. 21.1). One major dopaminergic pathway has its origin in the substantia nigra and projects to γ-aminobutyric acid (GABA)-ergic inhibitory interneurons in the corpus striatum through the nigrostriatal pathway (Ch. 24). This pathway modulates motor and behavioural function through ongoing projections to the thalamus and cortex. The corpus striatum in turn receives glutamatergic inputs from the cortex. Other major pathways connect the ventral tegmental area via the mesolimbic projections to the limbic region (especially the hippocampus) and via the mesocortical projections to the prefrontal cortex (the reward pathway; see Ch. 54). The limbic region also receives cortical afferents.
Several receptors for the neurotransmitter dopamine are found in the brain (see table of receptor families at end of Ch. 1). CNS dopamine receptors belong to two families: D1-like (which includes subtypes D1 and D5 that are coupled to a stimulatory G protein) and D2-like (which includes subtypes D2, D3 and D4 that are coupled to an inhibitory G protein). Postsynaptic D1 and D2 receptor subtypes are found in the dopaminergic pathways in the corpus striatum, limbic system, thalamus and hypothalamus. Postsynaptic D2 receptors are also present in the pituitary. Presynaptic D3 receptors are found on the dopaminergic neuronal terminals in the corpus striatum and limbic system, and their stimulation inhibits dopamine release in these areas. Postsynaptic D4 receptors are found in the limbic system and prefrontal cortex.
Schizophrenia is believed to involve interconnected abnormalities of glutamatergic and dopaminergic neurotransmission. However, it is uncertain whether reduced glutamatergic activity at N-methyl-D-aspartate (NMDA) receptors or increased dopaminergic neurotransmission at D2 receptors in the mesolimbic pathway is the primary abnormality. There are several pieces of evidence that support the involvement of defective glutamatergic and overactive dopaminergic neurotransmission in the genesis of schizophrenia:
 glutamate NMDA receptor antagonists (ketamine, phencyclidine) produce positive and negative symptoms, similar to those of schizophrenia,
glutamate NMDA receptor antagonists (ketamine, phencyclidine) produce positive and negative symptoms, similar to those of schizophrenia,In schizophrenia there is downregulation of D3 and D4 receptors in the prefrontal cortex, which may be responsible for the negative symptoms, and downregulation of D4 receptors in the limbic system. Dysfunction of other neurotransmitter systems utilising serotonin, GABA and neuropeptide Y may also be important in schizophrenia, but their precise roles are as yet unresolved.
Mania and bipolar disorder
Mania is a disorder of elevated mood that can occur alone (unipolar mania) or is more usually interspersed with episodes of depression (bipolar affective disorder or manic-depressive illness). Mild mania is termed hypomania. Sometimes, the fluctuations of mood are less marked, and the disorder is termed cyclothymia.
The onset of mania can be gradual or sudden, most often between the ages of 15 and 25 years, and varies in severity from mild elation, increased drive and sociability, to grandiose ideas, marked overactivity, overspending and socially embarrassing behaviour. Onset is usually early in adult life. Mania and bipolar disorder have (and share) a stronger genetic component than any other grouping of major psychiatric disorders.
Biological basis of bipolar disorder
The biological basis of bipolar disorder is less well understood than that for unipolar depression. Susceptibility genes have been identified that are shared with those for schizophrenia, but the environmental stressors that result in expression of the disorder are poorly understood. The dysregulation of neuronal function is probably triggered by altered expression of critical neuronal proteins, determined by the genetic predisposition. In bipolar disorder there is increased CNS monoamine neurotransmitter activity (particularly serotonin and dopamine) and reduced acetylcholine and GABA neurotransmission. These may all be important in orchestrating changes in neuronal function within the prefrontal cortex, visual association cortex and limbic circuitry.
The changes in neurotransmitter regulation produce functional disruption in the target neurons. Reduced neuronal levels of brain-derived neurotrophic factor (BDNF) may be important in the genesis of bipolar disorder (see also depression, Ch. 22). BDNF regulates several intracellular signal transduction pathways, and dysregulation of these pathways may produce the neuroplastic changes (especially synaptic plasticity) and neuronal cell loss that are features of bipolar disorder.
Antipsychotic drugs
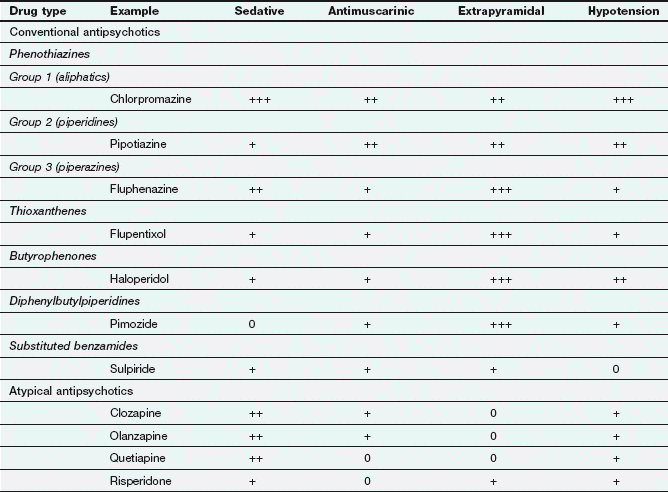
Antipsychotic drugs (also known as neuroleptics or major tranquillizers) have a common mechanism for their beneficial clinical effects, but belong to various chemical classes that differ in their propensity to cause sedation, and antimuscarinic or extrapyramidal effects (Table 21.2). They are commonly considered in two groups that differ in their unwanted effects: the conventional and atypical antipsychotics.
Conventional antipsychotic drugs
Mechanism of action and effects
The antipsychotic action of all conventional (or classical) antipsychotic drugs arises primarily from their antagonism of CNS dopamine D2 receptors in the mesolimbic pathway. High affinity for the family of D2 receptors is a common feature of all conventional antipsychotics, and the affinity of the drug for these receptors correlates well with its effective dose. Conventional antipsychotics have a higher affinity than dopamine for D2 receptors, and dissociate slowly from the receptor. At least 65% D2 receptor occupancy in the mesolimbic system is required for clinical benefit during long-term treatment of psychotic disorders. However, conventional antipsychotics also have D2 receptor antagonist activity in other CNS pathways, and 80% or more D2 receptor occupancy in the striatum will produce extrapyramidal unwanted effects (see below).
Many conventional antipsychotics also block serotonin 5-HT2A and 5-HT2C receptors, actions that may contribute to their clinical effects in suppressing negative symptoms. Antagonist activity at other receptors, including α1-adrenoceptors and histamine H1 receptors, does not influence their efficacy in psychotic illness but can produce unwanted effects (in which respect they resemble tricyclic antidepressants; Ch. 22). The severity of these unwanted effects varies considerably among the different drugs.
Clinical improvement with antipsychotic drugs develops slowly, despite an immediate antagonist action at dopamine receptors. There is increasing evidence that these drugs modulate complex intracellular pathways that affect neuroplasticity. This leads to changes in synaptic connections in areas of the brain known to be involved in psychotic illness which may be important for their long-term benefit. Clinically useful effects produced by antipsychotic drugs include the following.
 A depressant action on conditioned responses and emotional responsiveness: in psychoses this is particularly helpful for the management of thought disorders, abnormalities of perception and delusional beliefs.
A depressant action on conditioned responses and emotional responsiveness: in psychoses this is particularly helpful for the management of thought disorders, abnormalities of perception and delusional beliefs. A sedative action, which is useful for the treatment of restlessness and confusion: sensory input into the reticular activating system is reduced by inhibition of collateral fibres from the lemniscal pathways. Spontaneous activity is preserved but arousal stimuli produce less response.
A sedative action, which is useful for the treatment of restlessness and confusion: sensory input into the reticular activating system is reduced by inhibition of collateral fibres from the lemniscal pathways. Spontaneous activity is preserved but arousal stimuli produce less response. An anti-emetic effect through dopamine receptor antagonist activity at the chemoreceptor trigger zone (CTZ), which is useful to treat vomiting, such as that associated with drugs (e.g. cytotoxics, opioid analgesics) and uraemia: some antipsychotic drugs are also effective in motion sickness, through muscarinic receptor blockade (Ch. 32).
An anti-emetic effect through dopamine receptor antagonist activity at the chemoreceptor trigger zone (CTZ), which is useful to treat vomiting, such as that associated with drugs (e.g. cytotoxics, opioid analgesics) and uraemia: some antipsychotic drugs are also effective in motion sickness, through muscarinic receptor blockade (Ch. 32). Antihistamine activity produced by histamine H1-receptor antagonism can be used for treatment of allergic reactions (Ch. 39).
Antihistamine activity produced by histamine H1-receptor antagonism can be used for treatment of allergic reactions (Ch. 39).Pharmacokinetics
Conventional antipsychotics are rapidly absorbed from the gut but most undergo extensive first-pass metabolism. For some drugs, the plasma concentrations of active drug (including metabolites) can vary up to 10-fold among individuals, but there is not a close relationship between plasma drug concentration and clinical response. Elimination is by metabolism in the liver. Several antipsychotic drugs, such as chlorpromazine, haloperidol, perphenazine and zuclopenthixol, are metabolised predominantly by the polymorphic enzyme CYP2D6. There is a relationship between the steady-state plasma concentrations of these drugs (and therefore propensity to unwanted effects) and the CYP2D6 genotype (Ch. 2). Sulpiride does not undergo first-pass metabolism and is largely eliminated unchanged by the kidney. The half-lives of the antipsychotics vary widely; for example, that of sulpiride is 6–8 h, while the half-life of pimozide is very long, at 2 days. Some antipsychotics, such as chlorpromazine and haloperidol, can be given by intramuscular injection for more rapid onset of action.
Since adherence to treatment is often poor in psychotic disorders, depot formulations of many antipsychotics have been developed. They are given by intramuscular injection as a prodrug – which is the active compound esterified to a long-chain fatty acid and dissolved in a vegetable oil – that slowly releases the drug for between 1 and 12 weeks (depending on the formulation). When given as a depot preparation, or by deep intramuscular injection, the doses used are smaller than those for oral treatment, due to the absence of first-pass metabolism. The half-lives given in the Compendium at the end of this chapter do not reflect the slow absorption rate-limited half-life of the depot form (see Ch. 2). Examples of depot preparations are flupentixol decanoate and zuclopenthixol decanoate.
Unwanted effects
The antipsychotic drugs differ mainly in the degree of associated or unwanted effects (Table 21.2).
 Extrapyramidal effects arise from D2 receptor blockade in the nigrostriatal pathways, and take various forms: acute dystonias (tongue protrusion, torticollis, oculogyric crisis) are most common after the first dose or first few doses in children and young adults. Akathisia (restlessness) usually follows large initial doses, while parkinsonism has a gradual onset over several weeks usually in adults or the elderly. Extrapyramidal effects (Ch. 24) occur in more than half of those being treated with conventional antipsychotics, but are usually reversible if the drug is stopped. With prolonged use (several months to years) and especially in the elderly, tardive dyskinesias or tardive dystonias can develop. These consist of choreoathetoid and repetitive orofacial movements which often persist when the drug is withdrawn. Their aetiology is uncertain: upregulation of D2 receptors may contribute, but damage to inhibitory GABAergic neurons and/or dysfunction in other neurotransmitter pathways is probably involved. Extrapyramidal effects are most common with piperazine phenothiazines (such as prochlorperazine), the butyrophenones (such as haloperidol) and depot preparations (Table 21.2).
Extrapyramidal effects arise from D2 receptor blockade in the nigrostriatal pathways, and take various forms: acute dystonias (tongue protrusion, torticollis, oculogyric crisis) are most common after the first dose or first few doses in children and young adults. Akathisia (restlessness) usually follows large initial doses, while parkinsonism has a gradual onset over several weeks usually in adults or the elderly. Extrapyramidal effects (Ch. 24) occur in more than half of those being treated with conventional antipsychotics, but are usually reversible if the drug is stopped. With prolonged use (several months to years) and especially in the elderly, tardive dyskinesias or tardive dystonias can develop. These consist of choreoathetoid and repetitive orofacial movements which often persist when the drug is withdrawn. Their aetiology is uncertain: upregulation of D2 receptors may contribute, but damage to inhibitory GABAergic neurons and/or dysfunction in other neurotransmitter pathways is probably involved. Extrapyramidal effects are most common with piperazine phenothiazines (such as prochlorperazine), the butyrophenones (such as haloperidol) and depot preparations (Table 21.2). Drowsiness and cognitive impairment can occur as a result of histamine and dopamine receptor antagonism.
Drowsiness and cognitive impairment can occur as a result of histamine and dopamine receptor antagonism. Galactorrhoea, with gynaecomastia, amenorrhoea in women, impotence in men and reduced bone mineral density. These can arise when more than 70% D2 receptor occupancy in hypothalamic pathways produces hyperprolactinaemia and reduced gonadotrophin secretion. Antimuscarinic activity and α1-adrenoceptor antagonism also contribute.
Galactorrhoea, with gynaecomastia, amenorrhoea in women, impotence in men and reduced bone mineral density. These can arise when more than 70% D2 receptor occupancy in hypothalamic pathways produces hyperprolactinaemia and reduced gonadotrophin secretion. Antimuscarinic activity and α1-adrenoceptor antagonism also contribute. Antimuscarinic effects: peripheral antimuscarinic actions include dry mouth, constipation, micturition difficulties, blurred vision and reduced sexual arousal (Ch. 4). CNS muscarinic receptor blockade predisposes to acute confusional states.
Antimuscarinic effects: peripheral antimuscarinic actions include dry mouth, constipation, micturition difficulties, blurred vision and reduced sexual arousal (Ch. 4). CNS muscarinic receptor blockade predisposes to acute confusional states. Postural hypotension, nasal stuffiness and impaired erection and ejaculation in men due to α1-adrenoceptor antagonism.
Postural hypotension, nasal stuffiness and impaired erection and ejaculation in men due to α1-adrenoceptor antagonism. Hypothermia as a consequence of depressed hypothalamic function. Altered serotonergic neuronal activity may be responsible.
Hypothermia as a consequence of depressed hypothalamic function. Altered serotonergic neuronal activity may be responsible.< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree