14 The liver and biliary tract
The liver
Anatomy
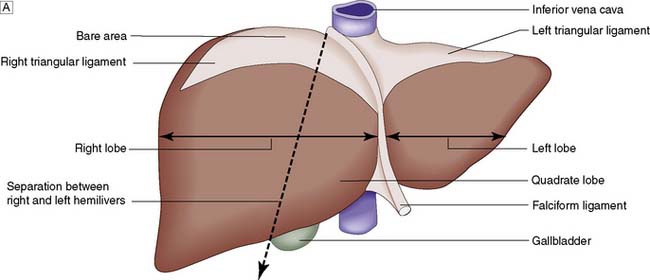
Topographically, the liver is divided by the attachment of the falciform ligament into right and left lobes; fissures on its visceral surface demarcate two further lobes, the quadrate and caudate (Fig. 14.1A). However, it is the liver segmental anatomy, as defined by the distribution of its blood supply, that is important to the surgeon.
Segmental anatomy
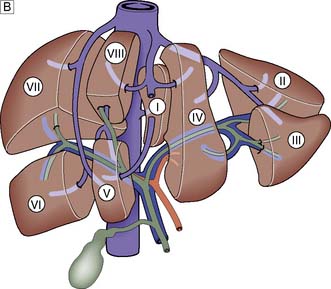
The portal vein and hepatic artery divide into right and left branches in the porta hepatis. Occluding either branch at surgery produces an easily visible line of demarcation that runs from the gallbladder bed behind and to the left of the inferior vena cava, thus separating the two hemilivers. Each hemiliver is further divided into four segments corresponding to the main branches of the hepatic artery and portal vein. In the left hemiliver, segment I corresponds to the caudate lobe, segments II and III to the left lobe (or left lateral section), and segment IV to the quadrate lobe. The remaining segments (V–VIII) comprise the right hemiliver (Fig. 14.1B).
Blood supply and function
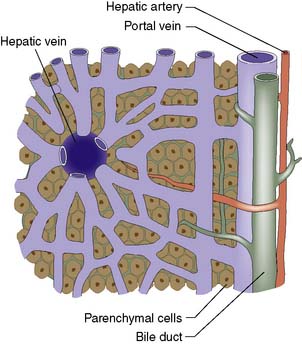
The liver normally receives 1500 ml of blood per minute and has a dual blood supply, 75% coming from the portal vein and 25% from the hepatic artery, which supplies 50% of the oxygen requirements. The principal venous drainage of the liver is by the right, middle and left hepatic veins, which enter the vena cava (Fig. 14.1B). In 25% of individuals, there is an inferior right hepatic vein, and numerous small veins drain direct into the vena cava from the caudate lobe (segment I). The functional unit of the liver is the hepatic acinus. Sheets of liver cells (hepatocytes) one cell thick are separated by interlacing sinusoids through which blood flows from the peripheral portal tract into the hepatic acinus to the central branch of the hepatic venous system. Bile is secreted by the liver cells and passes in the opposite direction along the small canaliculi into interlobular bile ducts located in the portal tracts (Fig. 14.2).
Summary Box 14.1 Surgical anatomy
• The liver is divisible into right and left hemilivers (each having four segments) using a line running from the gallbladder fossa to the inferior vena cava
• Each hemiliver receives a branch of the hepatic artery and portal vein; 75% of liver blood flow and 50% of its oxygen supply are provided by the portal vein
• The hepatocytes are arranged in lobules, each of which has a central branch of the hepatic vein and peripheral portal tracts (containing a branch of the hepatic artery, portal vein and bile duct)
• Liver anatomy allows the surgeon to perform right hepatectomy, left hepatectomy and extended right hepatectomy (i.e. resecting all of the liver to the right of the falciform ligament). Resection of individual segments is also possible.
Jaundice
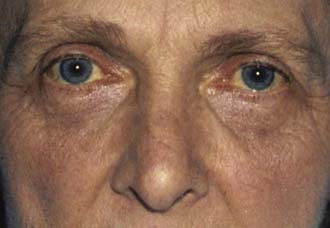
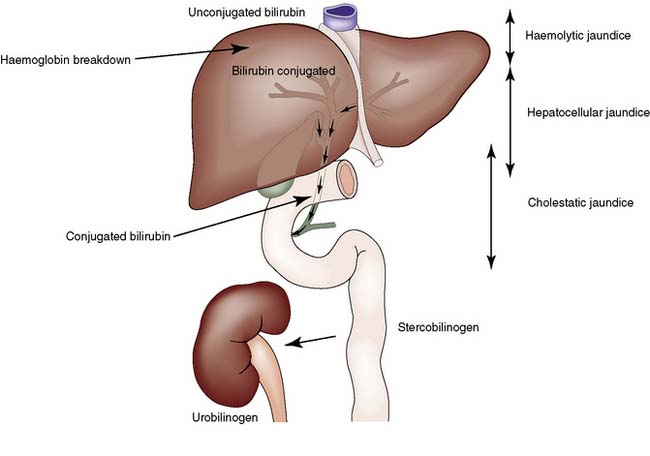
Jaundice is caused by an increase in the level of circulating bilirubin and becomes obvious in the skin and sclera when levels exceed 50 μmol/l (Fig. 14.3). It may result from excessive destruction of red cells (haemolytic jaundice), from failure to remove bilirubin from the blood stream (hepatocellular jaundice), or from obstruction to the flow of bile from the liver (cholestatic jaundice) (Fig. 14.4). Congenital non-haemolytic hyperbilirubinaemia (Gilbert’s syndrome) is a relatively rare cause of jaundice due to defective bilirubin transport; the jaundice is usually mild and transient, and the prognosis is excellent.
To the surgeon, the most important type of haemolytic jaundice is that caused by hereditary spherocytosis, in which splenectomy may be necessary (Ch. 15). Haemolytic jaundice may also occur after blood transfusion and after operative or accidental trauma, when haematoma formation produces a pigment load that exceeds hepatic excretory capacity.
Diagnosis
History and clinical examination
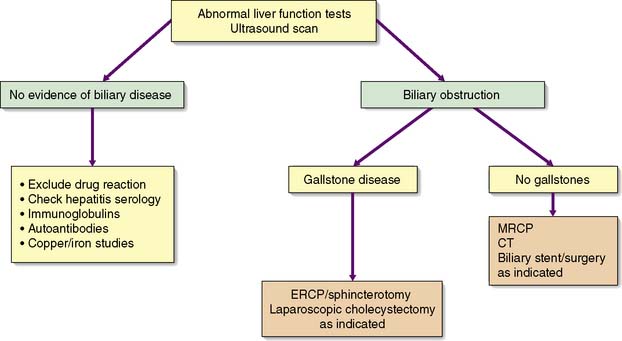
An accurate, rapid diagnosis of the cause of jaundice allows prompt institution of appropriate treatment (Fig. 14.5). The age, sex, occupation, social habits, drug and alcohol intake, history of injections or infusions, and general demeanour of the patient must be considered. A history of intermittent pain, fluctuant jaundice and dyspepsia suggests calculous obstruction of the common bile duct, whereas a history of weight loss and relentless progressive jaundice favours a diagnosis of neoplasia. Obstructive jaundice is likely if there is a history of passage of dark urine and pale stools, and if the patient complains of pruritus (owing to an inability to secrete bile salts into the obstructed biliary system). Hepatocellular jaundice is likely if there are stigmata of chronic liver disease, such as liver palms, spider naevi, testicular atrophy and gynaecomastia. The abdomen must be examined for evidence of hepatomegaly or gallbladder distension, and for signs of portal hypertension such as splenomegaly, ascites and large collateral veins (caput medusae) in the abdominal wall.
Radiological investigations
Congenital abnormalities
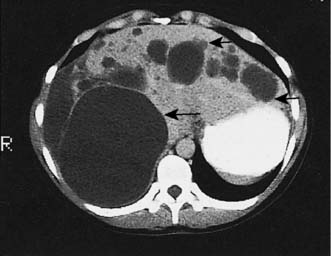
Up to 5% of the population has simple liver cysts. They are lined by biliary epithelium and contain serous fluid, but never communicate with the biliary tree. They rarely produce symptoms, are associated with normal liver function, and on ultrasound or CT have no discernible wall (Fig. 14.6). In the few patients who develop symptoms, cysts tend to recur following aspiration, and sclerosis by alcohol injection is of little value for large symptomatic cysts. Surgical management consists of deroofing and may be undertaken by laparoscopic means. Polycystic disease is a rare cause of liver enlargement and may be associated with polycystic kidneys as an autosomal dominant trait. In symptomatic patients, it may be necessary to combine a deroofing procedure with hepatic resection or to consider liver transplantation.
Summary Box 14.2 Jaundice
• Jaundice is a yellowish discoloration of the tissues that becomes clinically apparent when serum bilirubin levels exceed 50 μmol/l (normal < 20 μmol/l)
• It may be due to excessive haemolysis, hepatic insufficiency or cholestasis; cholestatic (obstructive) jaundice is the type encountered in surgical practice
• The two most common causes of surgical obstructive jaundice are cancer of the head of the pancreas and stones in the common bile duct (choledocholithiasis)
• In cholestatic jaundice, the bilirubin has been conjugated by the hepatocytes and is therefore soluble in water and can be excreted in the urine; patients with obstructive jaundice typically have dark urine and pale stools and may have pruritus (thought to be due to the accumulation of bile salts)
• Obstructive jaundice is characterized by elevated serum alkaline phosphatase levels in addition to hyperbilirubinaemia, and may be accompanied by modest elevations in transaminase (aminotransferase) levels, reflecting liver damage.
Liver trauma
After the spleen, the liver is the solid organ most commonly damaged in abdominal trauma, particularly following road traffic accidents. Stab injuries and gunshot wounds of the liver are also increasing in incidence. These are considered in Chapter 7.
Hepatic infections and infestations
Hydatid disease
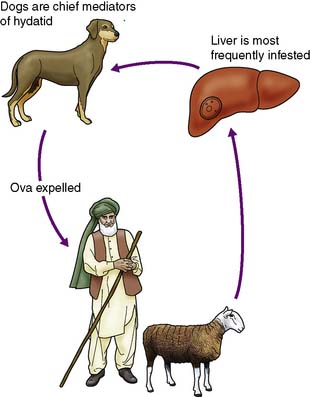
This less common infestation is caused in humans by one of two forms of tapeworm, Echinococcus granulosus and E. multilocularis. The adult tapeworm lives in the intestine of the dog, from which ova are passed in the stool; sheep or goats serve as the intermediate host by ingesting the ova whereas humans are accidental hosts (Fig. 14.7). The condition is most common in sheep- and goat-rearing areas. Ingested ova hatch in the duodenum and the embryos pass to the liver through the portal venous system. The wall of the resulting hydatid cyst is surrounded by an adventitial layer of fibrous tissue and consists of a laminated membrane lined by germinal epithelium, on which brood capsules containing scolices develop.
Portal hypertension
Portal hypertension is caused by increased resistance to portal venous blood flow, the obstruction being prehepatic, hepatic or posthepatic (Table 14.1). Rarely, it results primarily from an increase in portal blood flow. The normal pressure of 5–15 cmH2O in the portal vein is consistently exceeded (above 25 cmH2O). Portal vein thrombosis is a rare cause and is most commonly due to neonatal umbilical sepsis. The most common cause of portal hypertension is cirrhosis resulting from chronic liver disease and is characterized by liver cell damage, fibrosis and nodular regeneration. The fibrosis obstructs portal venous return and portal hypertension develops. Arteriovenous shunts within the liver also contribute to the hypertension.
Table 14.1 Causes of portal hypertension
| Obstruction to portal flow: |
| Prehepatic |
| Intrahepatic |
| Posthepatic |
| Increased blood flow (rare) |
Effects of portal hypertension
As a result of gradual chronic occlusion of the portal venous system, collateral pathways develop between the portal and systemic venous circulations. Portosystemic shunting occurs at three principal sites (Fig. 14.8). The most important is the development of varices in the submucosal plexus of veins in the lower oesophagus and gastric fundus. Oesophageal varices may rupture, to cause acute massive gastrointestinal bleeding in about 40% of patients with cirrhosis. The initial episode of variceal haemorrhage is fatal in about one-third of patients, and recurrent haemorrhage is common. Bleeding from retroperitoneal and periumbilical collaterals (‘caput medusae’) is troublesome during abdominal surgery, and collaterals may develop and cause bleeding at the site of stomas. Anorectal varices are not uncommonly found at proctoscopy but rarely cause bleeding.
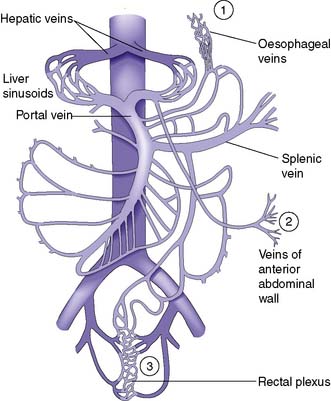
Fig. 14.8 The portal venous system.
Sites of portosystemic shunting are marked 1–3. Retroperitoneal communications also exist.
Clinical features
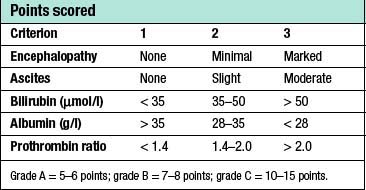
Patients with cirrhosis frequently develop anorexia, generalized malaise and weight loss. Clinical manifestations include hepatosplenomegaly, ascites, jaundice and spider naevi. Slurring of speech, a flapping tremor or dysarthria may point to encephalopathy, and this may be precipitated or intensified by the accumulation of blood in the gastrointestinal tract. The serum bilirubin may be elevated and the serum albumin depressed. Anaemia may be present and the leucocyte count raised (or depressed if there is hypersplenism). The prothrombin time and other indices of clotting may be abnormal. Clinical and biochemical parameters are used as the basis of the modified Child’s classification (Table 14.2). Patients allocated to grade A have a good prognosis, whereas those in grade C have the worst prognosis.
Table 14.2 Assessment of patients with portal hypertension using a modification of Child’s grading system
Acute variceal bleeding
Patients presenting with acute upper gastrointestinal bleeding are examined for evidence of chronic liver disease (EBM 14.1). The key investigation during an episode of active bleeding is endoscopy. This allows the detection of varices and defines whether they are or have been the site of bleeding. It is important to remember that peptic ulcer and gastritis are common complaints that occur in 20% of patients with varices.
14.1 Variceal bleeding in cirrhosis: assessment and prophylaxis
Jalan R, Hayes PC, British Society of Gastroenterology. Gut 2000; 46 suppl 3–4:III 1–III 15.
Management
The priorities in the management of bleeding oesophageal varices are summarized in Table 14.3.
Table 14.3 Priorities in the management of bleeding oesophageal varices
| Active resuscitation |
| Assessment of coagulation status |
| Urgent endoscopy |
| Control of bleeding |
| Treatment of hepatocellular decompensation |
| Treatment/prevention of portosystemic encephalopathy |
| Prevention of further bleeding from varices |
Endoscopy and control of bleeding
Endoscopy will reveal tortuous varices in three columns most prominent in the lower third of the oesophagus. Haemorrhage usually occurs from varices at the lowest few centimetres of the oesophagus. Rarely, bleeding occurs from varices in the gastric fundus. Although the synthetic form of somatostatin, octreotide, can be used to lower portal venous pressure and arrest bleeding, the injection of a sclerosant such as ethanolamine, or the application of bands is now used to arrest the bleeding at endoscopy (EBM 14.2). If haemorrhage is torrential and prevents direct injection, balloon tamponade may be used to stop the bleeding. The four-lumen Minnesota tube (Fig. 14.9) has largely replaced the three-lumen Sengstaken–Blakemore tube. The four lumina allow:
• aspiration of gastric contents
• compression of the oesophagogastric varices by the inflated gastric balloon
• compression of the oesophageal varices by the inflated oesophageal balloon
• aspiration of the oesophagus and pharynx to reduce pneumonic aspiration.