HEPATIC BLOOD FLOW AND PRESSURE
The liver receives its blood supply from two different sources. Approximately 2/3 or 1000 mL/min of the liver’s blood supply, is venous blood draining from the stomach, the small and large intestines, the pancreas, and the spleen. This blood comes to the liver via the portal vein. Because it is venous blood, it is poorly oxygenated but has a rich supply of nutrients, including glucose, which
the liver can convert into glycogen and immediately store. The blood may also contain intestinal bacteria, toxins, and ingested drugs. The other 1/3 of blood for the liver enters via the hepatic artery at a flow rate of approximately 500 mL/min. This is arterial blood and well saturated with oxygen. After perfusing the liver, both blood sources drain into the liver capillaries, called
sinusoids. From the sinusoids, blood drains into a central vein in each lobule and from there into the hepatic vein. The hepatic vein empties into the inferior vena cava.
In healthy individuals, there is virtually no resistance to the flow of blood in the portal vein. As a result, blood pressure in the portal venous system is low, approximately 3 mm Hg. Blood flows easily out of the liver into the vena cava as well, where pressure is nearly 0 mm Hg.
METABOLIC FUNCTIONS OF THE LIVER
Metabolism refers to the cellular processes that occur when basic food molecules (sugars, amino acids, and fatty acids) are built into cell structures or energy stores and are broken down later to run cell functions. The buildup of cell
structures and energy stores is called
anabolism; the breakdown is called
catabolism. The cells of the liver are key components in the interplay between anabolism and catabolism.
Glucose Handling by the Liver
After glucose is digested and absorbed into the bloodstream, it is delivered to all cells of the body to be used as an energy source. As discussed in
Chapter 16, insulin is required for glucose to gain entry to most cells. If glucose is unnecessary for immediate energy, it can be stored in cells as glycogen. The liver is especially capable of storing large amounts of glucose as glycogen. Because the liver can store glycogen, it acts as a glucose buffer for the blood. When glucose levels rise in the blood, the liver’s conversion of glucose to glycogen and the storage of glycogen increase. Glycogen formation, called
glycogenesis, occurs in the
absorptive phase of digestion, which is the period soon after a meal when glucose levels are high. Glycogenesis is insulin dependent. By increasing the conversion and storage of glucose in times of excess, the liver returns plasma glucose levels toward normal.
In times of fasting or between meals, the breakdown of glycogen to glucose occurs in the liver, again serving to normalize circulating levels of glucose. The breakdown of glycogen is called
glycogenolysis. In addition, when glucose levels decrease between meals, the liver initiates
gluconeogenesis (the new formation of glucose) to keep blood glucose levels constant. Gluconeogenesis is accomplished in the liver by conversion of amino acids to glucose after deamination (removal of the amino group) and by conversion of glycerol, a product of fatty acid breakdown, to glucose. The breakdown of glycogen and the formation of glucose occur in the
postabsorptive phase of digestion, the time between meals when external food sources are not readily available. The postabsorptive stage of digestion is under the control of the pancreatic hormone glycogen and other gastrointestinal hormones, as discussed in
Chapter 15.
Amino Acid Handling by the Liver
After digestion, amino acids enter all cells and are converted to proteins to be used by the cells to make either enzymes or structural components such as ribosomes, collagen, or muscle contractile proteins. Although a variety of organs (including the kidney and intestinal mucosa) participate in the storage of extra amino acids as proteins, the liver is the major storage tissue for protein. When amino acids are needed, the breakdown of stored protein occurs, and free amino acids are liberated. A decrease in plasma amino acids below a certain level triggers the breakdown of stored proteins.
All cells, including liver cells, have limits on how much protein they can store. When no further amino acids can be stored as protein, the liver deaminates the extra amino acids and either uses the products as energy or changes them into glucose, glycogen, or fatty acids. These substances can be stored in
the liver—glucose as glycogen and fatty acids as triglycerides (fat). Fatty acids can also be stored in other cells of the body, especially adipose tissue.
During deamination of amino acids, ammonia is released. It is almost entirely converted in the liver to urea, which is then excreted by the kidneys.
Fatty Acid Handling by the Liver
Nearly all digested fats are absorbed into the lymphatic circulation as chylomicrons—conglomerates of triglycerides, phospholipids, cholesterol, and lipoprotein. The chylomicrons are delivered by the lymph to the thoracic duct, where they join the systemic circulation. Triglycerides are subsequently changed back into fatty acids and glycerol by enzymes in the walls of all capillaries, especially the capillaries that serve the liver and the adipose tissue. From the capillaries, fatty acids and glycerol can diffuse into most cells.
Once inside the liver and other cells, fatty acids and glycerol again combine to form triglycerides. Triglycerides are stored until needed during the postabsorptive stage. At this time they may be metabolized back to glycerol and free fatty acids. Glycerol and fatty acids can enter the Krebs cycle to produce ATP, so that cells are provided with energy. Elevations in the hormones glucagon, cortisol, growth hormone, and the catecholamines signal cells to break down stored triglycerides into free fatty acids and glycerol.
Instead of directly entering the Krebs cycle, some glycerol and free fatty acids may be used by the liver to produce new glucose. This can result in the production of ketones when triglyceride breakdown is excessive. The brain itself cannot use free fatty acids directly for energy production. Therefore, the liver’s conversion of fats to glucose (gluconeogenesis) is essential for supporting the energy needs of the brain when glucose levels are low.
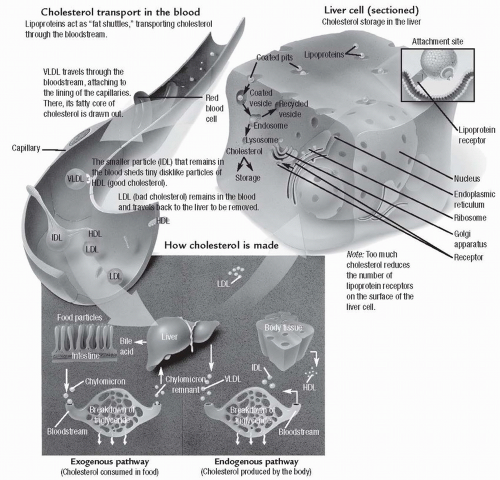
Cholesterol Handling by the Liver
Cholesterol is a lipid substance produced by the liver and used in the digestion of fat. During digestion, cholesterol is packaged with bile salts, phospholipids, and the triglycerides into small suspensions called micelles. Once the triglycerides are suspended as micelles, they can be digested by pancreatic enzymes and absorbed into the bloodstream. The cholesterol from the micelles is recirculated to the liver. The liver metabolizes some of the cholesterol and recycles it to be used again in digestion. The remainder is complexed with phospholipids and released into the bloodstream as
lipoproteins. As lipoproteins, cholesterol is carried to body cells to be used for the production of cell membranes, intracellular structures, and steroid hormones. High levels of two types of lipoproteins, low-density lipoprotein (LDL) and very low-density lipoprotein (VLDL), suggest that the liver is handling high amounts of cholesterol. These types of lipoproteins may injure cells, including the endothelial cells lining the arteries, by releasing oxygen free radicals and high-energy electrons during their metabolism. High-density lipoprotein (HDL), on the other hand, carries
cholesterol away from cells to the liver and protects against arterial disease.
Figure 17-1 illustrates the process of cholesterol transport and storage.
BILE SECRETION
Bile is made by all hepatocytes and consists of water, bile salts, bilirubin, cholesterol, fatty acids, lecithin, and electrolytes. Except for water, the most abundant substance in bile is bile salt. Bile salts are synthesized in the liver from cholesterol that has either been delivered to the liver from the small intestine or synthesized directly by the liver in the process of fat metabolism. All hepatic cells participate in making bile and each secretes bile into the small bile
canaliculi that surround all liver cells. The canaliculi empty into progressively larger ducts that ultimately join into the
hepatic duct and
common bile duct. These ducts deliver bile either to the gallbladder for storage or directly into the intestine.
Bile salts function in the digestion of fat (see
Chapter 15) and are normally recycled after use in the small intestine. Without bile, as much as 40% of fats in the diet would not be absorbed across the intestine and so would be lost in the stool. The absorption of fat-soluble vitamins across the small intestine would be similarly affected. For example, without bile, a vitamin-K deficit would occur and be apparent in less than a week. Without adequate vitamin K, blood coagulation would be impaired.
The liver also functions in the handling of another component of bile, bilirubin. Bilirubin is formed as an end product of hemoglobin breakdown and must be metabolized by the liver for it to be excreted.
METABOLIC BIOTRANSFORMATION
The liver has an important role in transforming biologic substances that may be toxic at high levels or that cannot be excreted from the body without transformation. Substances acted upon in this manner by the liver may include both those ingested by an individual and those produced by the body itself. Examples of substances that are transformed by the liver include bilirubin, various hormones, drugs, and toxins. Metabolic biotransformation is also referred to as metabolic detoxification.
Bilirubin Biotransformation
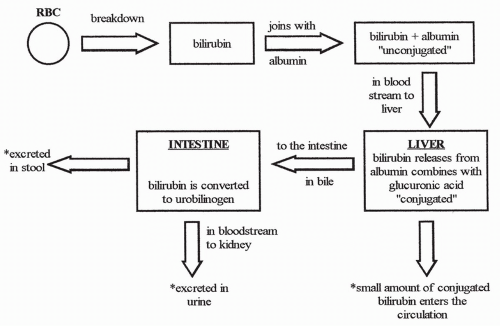
Bilirubin is a product of red blood cell breakdown. When a red blood cell has lived out its 120-day life span, the cell membrane becomes fragile and ruptures. Hemoglobin is released and is acted upon by circulating phagocytic cells to form free bilirubin. Free bilirubin binds to plasma albumin and circulates in the bloodstream to the liver.
Free bilirubin is considered unconjugated in that, although it is bound to albumin, the binding is reversible. Once in the liver, bilirubin releases from albumin and, because free bilirubin is lipid soluble, moves easily into the hepatocytes. Once inside the hepatocytes, bilirubin is rapidly bound to another substance, usually glucuronic acid, and is now considered conjugated. Conjugated bilirubin is water soluble, not lipid soluble.
Most conjugated bilirubin is actively transported into the bile canaliculi. From there it is delivered along with the other components of bile to the gallbladder or small intestine. A small amount of conjugated bilirubin does not go to the intestine as a bile component, however, but rather is absorbed back into the bloodstream. Therefore, in the bloodstream, there is always a small amount of conjugated bilirubin present, along with unconjugated bilirubin on its way to the liver.
Once in the intestine, conjugated bilirubin is acted upon by bacteria and changed into urobilinogen. Most urobilinogen enters the bloodstream and is excreted by the kidneys in the urine, some is excreted in the stool,
and some is recycled back to the liver in the enterohepatic (intestinal to liver) circulation.
Figure 17-2 shows the steps involved in the conjugation and excretion of bilirubin.
The conjugation of bilirubin is essential for its excretion. Without conjugation, bilirubin cannot be excreted by either the kidneys or the intestines. The handling of bilirubin by the liver is a form of metabolic detoxification. Without conjugation, unconjugated bilirubin would build up in the bloodstream to toxic levels.
Hormone Biotransformation
The liver inactivates or modifies many hormones of the body. It acts on steroid hormones, including cortisol, estrogen, testosterone, progesterone, and aldosterone, to make them water soluble rather than lipid soluble, allowing them to be excreted. If this biotransformation does not occur, these hormones tend to concentrate in the body and build up in tissues, especially adipose tissue.
Liver proteases inactivate or deaminate other hormones such as insulin, glucagon, and antidiuretic hormone (ADH, also called vasopressin). Thyroxine is deiodinated and inactivated. These actions allow the hormones to be excreted from the body.
Ammonia Biotransformation
Ammonia is a by-product of protein breakdown. It is transformed into urea in the liver and excreted in the urine. Without this liver function, ammonia levels build up in the blood and cause neurological dysfunction and possibly coma and death.
Drug and Toxin Metabolism
Drugs and toxins are often modified by the liver to be either inactivated or made water soluble by conjugation with another chemical compound. By these processes, the liver permits the body to excrete these substances. Without good liver function, many drugs and toxins accumulate in the body. Moreover, many of the chemical compounds used by the liver to conjugate lipid-soluble drugs and toxins, such as plasma proteins, are themselves synthesized by the liver. Therefore, they are in inadequate supply in the case of a poorly functioning liver.
Alcohol Handling by the Liver
Alcohol is an example of a drug that is primarily metabolized by the liver. Alcohol metabolism follows two pathways in the liver. The first pathway uses the enzyme alcohol dehydrogenase and results in the end product of acetaldehyde. Acetaldehyde is then changed to acetate and hydrogen ions. These reactions occur in the cytoplasm and the mitochondria of the hepatocyte.
The second metabolic pathway, called the microsomal ethanol oxidizing system (MEOS) pathway, named after the specific enzymes involved, occurs in the endoplasmic reticulum of the hepatocyte and is primarily used in the liver of individuals who have a long history of alcohol abuse. This pathway results in the production of acetaldehyde and free radicals. The free radicals and the acetaldehyde produced by either metabolic pathway are highly damaging to liver cells.
The MEOS pathway is also damaging to an individual because one of the enzymes required for running this pathway, cytochrome P450, is essential in the liver’s transformation of many other toxins and drugs and excess fat-soluble vitamins. If this enzyme is preferentially used to detoxify alcohol, it is unavailable for its other roles. Thus, long-term alcohol abusers are susceptible to damage from many different toxins and drugs, and to the toxic effects of some vitamins.
Another coenzyme in alcohol metabolism is nicotinamide adenine dinucleotide (NAD). This coenzyme is also required for many other metabolic processes, including running the Krebs cycle to metabolize nutrients, making ATP, and allowing the liver to perform gluconeogenesis. Without NAD, hypoglycemia and lactic acid accumulation may develop. Hypoglycemia is a significant problem for many long-term alcohol abusers who typically have poor diets. Lactic acid accumulation can contribute to gout because increased lactic acid decreases the renal excretion of uric acid.
CLOTTING FACTOR SYNTHESIS
The liver functions in the production of several clotting factors, including factors I (fibrinogen), II (prothrombin), and VII (proconvertin). Without adequate production of these substances, blood clotting is impaired and bleeding may be extensive. In addition, vitamin K is a fat-soluble vitamin required for the formation of these and other clotting factors. Because bile salts are required for across-the-gut absorption of all fat-soluble vitamins, liver dysfunction resulting in decreased synthesis or supply of bile to the intestine can also lead to bleeding problems.