http://evolve.elsevier.com/Edmunds/NP/
Therapeutic Overview
The goal of immunization is the eradication of disease. Numerous infectious diseases, many of which are potentially fatal, have been sharply curtailed worldwide through vigilant adherence to immunization strategies and public health control measures. In the United States, diphtheria, measles, polio, and tetanus are almost unknown. Children who are not vaccinated for religious, cultural, or other reasons are at risk for disease and increase societal risk by contributing to the pool of unvaccinated individuals who are capable of transmitting infection to susceptible and high-risk individuals.
According to the 2011 National Immunization Survey, reported in the September 6, 2012 issue of Morbidity and Mortality Weekly Report, immunization rates for many routine vaccines in 2011 were 90% or more among kids aged 19 months to 35 months. Coverage for the birth dose of hepatitis B increased from 64.1% in 2010 to 68.6% in 2011, coverage for the recommended two doses of hepatitis A vaccine increased from 49.7% to 52.2% in the same period, coverage for rotavirus vaccines increased from 59.2& to 67.3%, and coverage for the full series of Haemophilus influenzae type b (Hib) vaccine increased from 66.8% to 80.4%. Vaccination coverage remained above the Healthy People 2020 target of 90% for measles, mumps, and rubella (91.6%); poliovirus (93.9%); varicella (90.8%); and hepatitis B (91.1%). The immunization rates are highest among Asians and high-income children.
Immunization rates in Medicaid plan members ticked up in contrast to a decline of approximately 4% in children insured under commercial plans. One reason for this divergence suggested in a report from the National Committee for Quality Assurance is the persistence of a popular but discredited belief that vaccines cause autism spectrum disorders.
More than 1 in 20 public school kindergartners in eight states did not receive all the required vaccines for attendance, another analysis found. Ten states recorded exemption rate increases of about 1.5 percentage points or more over 5 years. Although health authorities have not determined an exemption threshold that could lead to outbreaks, they are concerned that some exemption rates are increasing beyond 5%. An online survey of 750 parents of children aged 6 and younger in the journal Pediatrics revealed that more than 1 in 10 refused to follow vaccination guidelines as advised by the government due to safety concerns. Researchers found that even 20% of parents whose children received the recommended vaccinations believe that it is safer to delay administering the vaccines.
Anatomy and Physiology: The Immune System
The first line of defense against disease consists of the skin, mucous membranes, body hair, and body secretions. The second line of defense is the inflammatory response, which is critical for the body’s survival when faced with stressors from the environment and is increasingly recognized as an important factor in a variety of acute and chronic diseases. The immune system is the third line of defense against the invasion of antigens.
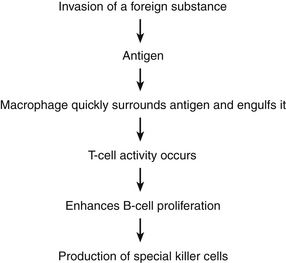
The main function of the immune system is to protect the body from damage caused by the introduction of a foreign substance. A number of stimuli can trigger the inflammatory response. These include infectious agents, ischemia, antigen–antibody interactions, and thermal or other injury. In many conditions, the cause of the inflammation is not known. The inflammatory response includes three phases: (1) acute, transient, local vasodilation and increased capillary permeability; (2) delayed, subacute infiltration of leukocytes and phagocytic cells; and (3) chronic proliferative tissue degeneration and fibrosis.
Organs of the immune system consist of primary and secondary organs. Primary organs are responsible for the development and storage of lymphocytes. The bone marrow and the thymus gland are the primary organs of the immune system. Secondary organs include lymph nodes, spleen, and Peyer’s patches. These secondary organs of the immune system entrap foreign substances, produce antibodies, and stimulate T-cell production, all with the main objective of destroying the antigen. All WBCs originate from a stem cell in the bone marrow. Stem cells first differentiate into myeloid and lymphoid cells. Myeloid cells differentiate into polymorphonuclear (PMN) leukocytes and into monocytes/macrophages. Lymphoid cells differentiate into B- and T-lymphocytes (Figure 70-1).
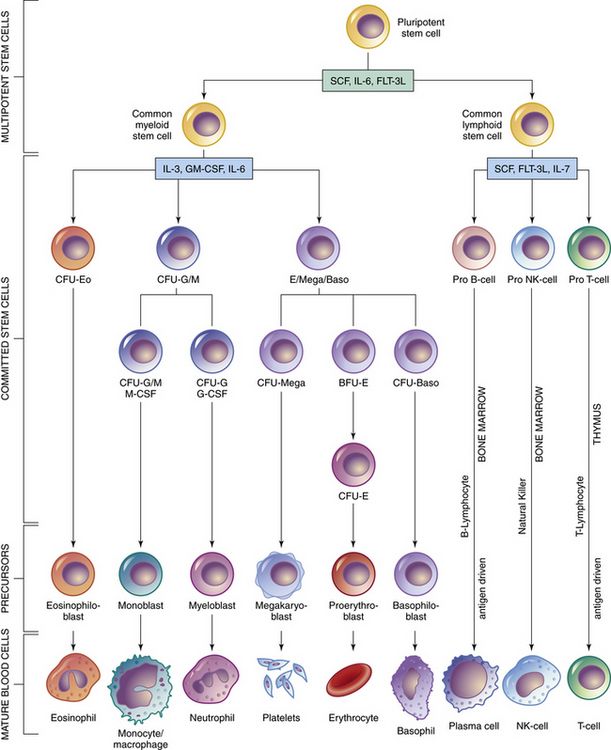
FIGURE 70-1 Bone marrow and stem cell systems. Probable pathways of differentiation, from the totipotential cell to mature blood cells. (PMN, Granulocytes.) (From McCance KL, Heuther SE: Pathophysiology, ed 5, St Louis, 2008, Mosby.)
Two types of nonspecific WBCs and two types of specific WBCs have been identified.
Nonspecific WBCS
The first type of WBC includes polymorphonuclear leukocytes, also called granulocytes (e.g., neutrophils, eosinophils, basophils, and mast cells). These are the most active cells and contain the largest number of immune cells in the body. They arrive first at a site of injury, infection, or inflammation and function in several ways. They phagocytize foreign substances and release chemotaxic substances that encircle the area of invasion, killing and preventing contamination by foreign substance into other areas; they also stimulate the release of antimicrobial substances that aid in the destruction of foreign material.
The second type of nonspecific WBC is the monocyte/macrophage. When monocytes are released into the bloodstream, they migrate to various tissue sites, where they differentiate (mature) and become macrophages. Macrophages serve three functions in the immune response. The first is to secrete biologically active compounds/molecules such as prostaglandins, interleukins, interferons, tumor necrosis factors, growth factors, proteins, and enzymes, which serve to provide host defense from specific antigens. The second is to remove excess dead or damaged antigens. The third is to engulf and present antigens to lymphoid cells for elimination. Macrophages are found in connective tissue (e.g., histocyte), the liver (e.g., Kupffer’s cells), alveolar tissue in the lung, and microglial cells in the nervous system. They are also found in the spleen, lymph nodes, and other organs.
Specific WBCs
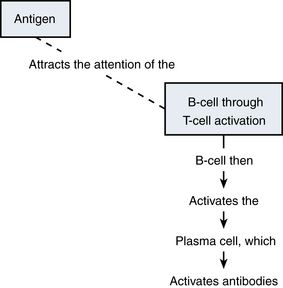
The specific WBCs consist of the two lymphocytes (B- and T-cells). These cells react with antigens to produce reactions that create a specific response that will destroy the antigen. These do not participate in nonspecific inflammatory responses.
Other Immune System Components
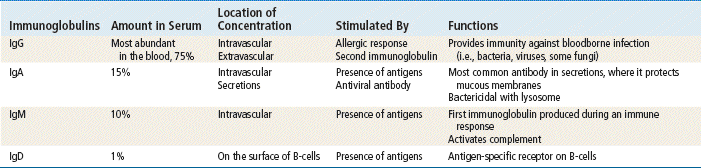
Three plasma protein systems are located in the plasma of blood, not inside a cell. These include the complement, clotting, and kinin systems. Each initiates a cascade of reactions, ending with potent biochemical mediators of the inflammatory response. The complement system is a nonspecific mediator of inflammation that is potent against bacterial infection. IgG or IgM usually initiates the cascade by forming an immune complex. The kinin system begins with bradykinin, which causes dilation of vessels, acts with prostaglandins to induce pain and increase vascular permeability, and is important in the prolonged phase of inflammation. Platelets stop bleeding and release serotonin, which has vascular effects similar to histamine. The clotting system is discussed in Chapter 25. Cytokines are glycoproteins, which are chemical messengers that modulate the immune response.
The Nonspecific Inflammatory Response
Two basic antiinflammatory actions occur: phagocytosis (i.e., ingestion of unwanted material) and secretion of cytokines that mediate the inflammatory response. A bewildering array of these substances with overlapping sources and functions have been identified.
Macrophages, mast cells, T-helper cells, natural killer cells, and others secrete many cytokines, including colony-stimulating factor interleukins, tissue necrosis factor, and interferon.
The inflammatory response begins when circulating proteins and blood cells come into contact with a stimulus. Neutrophils arrive at the site first and phagocytose (ingest) the particles that are causing the inflammation. The mast cells are already present in the loose connective tissues close to blood vessels. Monocytes and macrophages arrive and begin phagocytosis. Mast cells, monocytes, and macrophages release many substances called collectively mediators or facilitators of inflammation. The mast cell is the most important activator of the inflammatory response.
Mast cells immediately release substances from their granules, which cause immediate inflammation. These substances include histamine, neutrophil chemotactic factor, and eosinophil chemotactic factor. The mast cell also synthesizes and then releases leukotrienes and prostaglandins, which cause long-term inflammation (see Figure 34-2). These facilitators start a chain of reactions, thereby producing exudate that defends against infection and facilitates tissue repair and healing.
Whereas many mediators of inflammation are known, this discussion focuses on the prostaglandins, which are affected by aspirin and NSAIDs.
Prostaglandins cause increased vascular permeability and neutrophil chemotaxis (movement), and they induce pain. Increased vascular permeability allows diffusion of large molecule inflammatory substances across cell walls into the site of inflammation. Prostaglandins are made within the mast cell from arachidonic acid through the action of the enzyme cyclooxygenase (COX) and are classified into groups according to their structure. Prostaglandins E1 and E2 are active in the inflammatory response. Aspirin and NSAIDs act to block the enzyme COX from producing prostaglandins, thereby inhibiting inflammation (see Chapter 34 and Figure 34-2).
The Specific Immune Response
The immune response is activated through generation of humoral or cellular immunity. B-lymphocytes are the cells involved in antibody-mediated or humoral immunity. T-lymphocytes are the effectors for cell-mediated immunity. This response can be summarized as follows:
Mechanism of Action
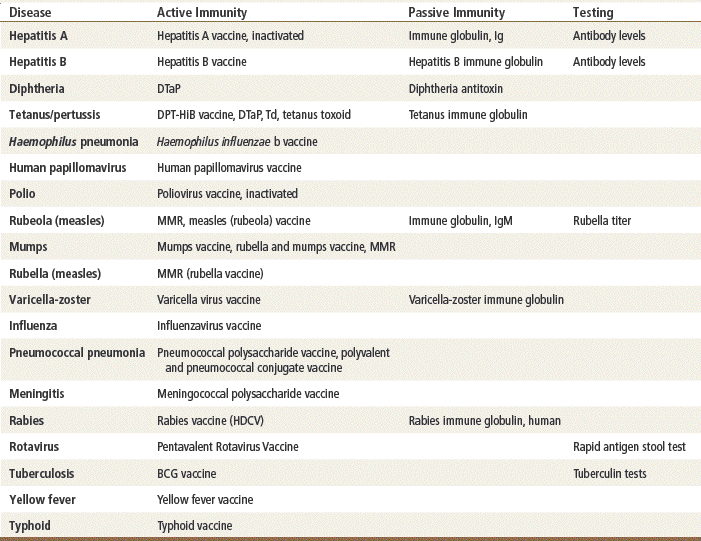
Immunizations act to confer immunity to a particular disease. There are two types of immunity: active and passive.
Therapeutic Overview
Active immunization involves the administration of all or a part of a microorganism to evoke a response. Antigens are taken from living or dead organisms, and small amounts are given intradermally or subcutaneously. This process stimulates the body’s immune response, and antibodies are stimulated to protect the immunized person from greater exposure to this particular disease-producing antigen. This immunity is retained for a prolonged period, thereby protecting the person from the disease whenever he or she may be exposed to that antigen. This immunity can be “boosted” at specific intervals.
Active immunization is accomplished with three different types of agents:
Passive Immunity
Passive immunity occurs when antibodies that one acquired from a human or an animal (with acquired immunity to a specific organism) are given to people who do not have immunity to the organism. Newborn infants achieve this naturally from their mothers through the placenta and through breastfeeding. It can also be achieved through injections of gamma globulins (for hepatitis protection) or antisera or antitoxins. This process temporarily provides the same protection as that given to a person who has achieved active acquired immunity. These antibodies naturally break down and are eliminated from the body. See Table 70-1 for a summary of the characteristics and function of immunoglobulins.
Antisera (i.e., antibodies of animal origin used to counteract the effects of a toxin) and human plasma are used after exposure. Very specific indications and guidelines govern the use of these products. They are not discussed in detail in this chapter. Contact your local health department or the CDC for guidelines concerning the use of antisera.
Incompetent immune systems do not develop active immunity in response to vaccines and toxoids. These patients need protection from infection. This protection can be accomplished through passive immunity, identification of the deficient immune mediators, and replacement of those mediators, or by giving these patients antiinfective drugs. Agents that are classified as immune mediators are agents such as interferon, interleukins, and immunoglobulin.
All immunizations contain several different components, such as the following:
Comparable vaccines made by different manufacturers may be used interchangeably, if used according to recommended guidelines. Available data suggest that adequate response occurs even when products from different manufacturers are used during the same series.
Figure 70-4 shows the recommended childhood immunizations generally used in primary care.

FIGURE 70-4 Recommended childhood immunization schedule for children aged 0 to 6 years.
Schedules for persons aged 7 to 18 years, catch-up for persons aged 4 months to 18 years, and adult immunizations are available at http://www.cdc.gov/vaccines/recs/schedules/default.htm. From Centers for Disease Control and Prevention, National Immunization Program: Recommended childhood and adolescent immunization schedule: United States 2011. http://www.cdc.gov/vaccines/schedules/downloads/child/0-6yrs-schedule-pr.pdf.
Treatment Principles
Evidence-Based Recommendations
Cardinal Points of Treatment
Guidelines concerning the use of immunizations are one of the most carefully studied areas of pharmacology. Joint guidelines for children are promulgated by the ACIP and the American Academy of Pediatrics (AAP). Guidelines are revised yearly as new products are introduced and recommendations for use of older products change; these guidelines determine the standard of care that all primary care providers are expected to provide. With the increase in the immunocompromised population and the increasing numbers of immigrants living in the United States who require catch-up vaccination, the greatest challenge for primary care providers is to master the exceptions to the recommended schedule. Figure 70-4 provides the recommendations for pediatric immunizations from the ACIP and the AAP. The latest updates are available on the CDC website.
Critical decisions to be made in immunizations include which products to give and when to give them. Before using any biologic, the health care provider should take all precautions known for the detection or prevention of allergic or any other adverse reaction. This should include a review of the patient’s history regarding possible sensitivity, the ready availability of epinephrine 1:1000 and other appropriate agents used for control of immediate allergic reactions, and a knowledge of the recent literature pertaining to the biologic to be used, including the nature of side effects and adverse effects that may follow its use.
Individuals with impaired immune responsiveness, whether because of the use of immunosuppressive therapy, a genetic defect, HIV/AIDS, or other causes, may have a reduced antibody response to active immunization procedures. Deferral of the administration of live vaccines may be considered in individuals receiving immunosuppressive therapy. Other groups should receive vaccines according to the usual recommended schedule.
Schedule for Immunization of Children
Both the CDC’s ACIP and the AAP Committee on Infectious Disease have issued immunization guidelines for children that are revised annually and published each January. Recommendations from these two bodies may vary slightly, and these variances are noted in a joint Recommended Vaccine Schedule, which was last issued in February 2012. Data from the 2006-2009 National Immunization Survey-Teens revealed that the number of children aged 13 to 17 who were current on the tetanus, diphtheria, whooping cough, meningitis, and human papillomavirus vaccines increased from 10% in 2006 to nearly 42% in 2009. The CDC report also concluded that HPV vaccination is still lagging, although the number of girls who completed the three doses increased from 18% in 2006 to 27% in 2009.
Immunization of Adult and Elderly Persons
Recommendations for adult immunization historically were lacking. However, with the recognition of increased pertussis, pneumococcus, and other vaccine-preventable diseases in adults, the CDC issued the first schedule for adult immunizations in October 2002, which is updated annually. The most recent schedule was released in February 2012. A 2012 CDC Morbidity and Mortality Weekly Report concluded that the number of U.S. adults who received routinely recommended vaccinations between 2008 and 2010 remained low. The report found that tetanus, diphtheria, and acellular pertussis coverage increased from 6.6% in 2009 to 8.2% in 2010, while pneumonia, hepatitis A, and hepatitis B rates did not change significantly. There has been substantial direct to consumer advertising to grandparents to get these immunizations to protect their newborn grandchildren.
At-Risk and Postexposure Patients
Certain populations are considered to be at risk and require modification of the routine vaccination schedule. Details for these exceptions may be found on the CDC website.
Some of the important exceptions are summarized as follows:
Principles of Administration of Vaccines
Numerous myths exist about contraindications to administration of vaccines. For all currently available products, the following principles apply:
All immunizing agents have specific contraindications that will be discussed separately.
Notification of Risks and Benefits of the Vaccine
The National Childhood Vaccine Injury Act of 1986 mandates the notification of patients and parents of the risks and benefits of individual vaccines. This legislation requires the distribution of standardized information to these individuals. A simplified version of information pamphlets was approved by federal legislation in 1993 and is available from vaccine manufacturers, the CDC, and most state health departments.
The legislation requires health care providers who administer vaccines to keep permanent records of all immunizations given, along with specific information about the manufacturer of the product and the lot number of all doses. In addition, the provider is required to report occurrences of events suspected to be the result of vaccine by using a mechanism titled the Vaccine Adverse Event Reporting System (VAERS). Reports may be made online at http://vaers.hhs.gov. The specifics of reportable events for each agent are discussed separately. In addition, this act established a Vaccine Injury Compensation Table that determined the injuries, disabilities, and conditions for which compensation may be made.
How to Monitor
Vaccines that are administered as recommended induce protective immunity in more than 95% of recipients. It is not recommended or necessary to obtain serum titers to document immunity. A U.K. study in the journal Archives of Disease in Childhood found that anaphylaxis cases in children following immunization were extremely rare.
For patients with signs and symptoms suggesting an immunoglobin E–mediated reaction to a vaccine or its components, allergy testing by a specialist may be indicated, especially when future doses of the suspect vaccine(s) will be needed. A four-step protocol published by the Hypersensitivity Study Group in 2009 facilitates this process, which should preferably be performed by an allergist, using the specific vaccine, from the same maker, that is suspected of causing the reaction.
Decisions about whether the patient should have additional vaccination should be based on patient-specific risk/benefit analysis as guided by the algorithm. Some of the options for revaccination include withholding other doses of suspected or implicated vaccines for patients who have serologic evidence of immunity, who are at low risk for disease, who have serious complications from disease, or who are at risk for life-threatening complications from the vaccine.
A CDC analysis of medical records of 550 patients with Guillain-Barré syndrome showed that none of them experienced flare-ups in the 2 months after receiving vaccines, including flu shots. Only one patient experienced a flare-up within a year of vaccination. This information should be provided to individuals with a history of Guillain-Barré syndrome who would like to receive vaccinations to prevent illness.
The question of whether vaccines cause autism has remained in the minds of the public despite numerous scientific studies concluding that they do not. A panel of the Institute of Medicine once again concluded that the MMR vaccine does not cause autism despite complaints from some parents’ groups.
But vaccines are not without risk. There are risks to getting the vaccine for chickenpox that can arise years after vaccination. People who have had the vaccine can develop pneumonia, meningitis, or hepatitis years later if the virus used in the vaccine reawakens due to an unrelated health problem, like cancer, that has compromised their immune system. However, the same problems are far more likely in patients who are infected naturally at some point in their lives with chickenpox, since varicella-zoster, the virus that causes chickenpox, can live dormant in nerve cells for decades and present later as shingles.
The government asked the Institute of Medicine in 2012 to review the known risks of different kinds of vaccines to help guide decisions about compensation for those who claim to have been injured by vaccines. Legislation passed in 1986 by Congress basically absolved vaccine makers of the risks of being sued for vaccine injuries to encourage companies to continue to manufacture vaccines and force those who suffer some type of injury they believe is related to vaccines to petition the government for compensation. The government generally restricts compensation to cases involving children who have injuries that scientists believe might plausibly have been caused by vaccination, including seizures, allergic reactions, fainting, inflammation, and temporary joint pain. But legal and legislative battles have been fought for years over whether to expand this list because of the concern about vaccines and autism. Much of this concern was due to a fraudulently reported study that was later retracted. Rather than vaccines causing autism, it has been suggested that many children found to be injured by vaccination have an immune or metabolic problem that becomes obvious after vaccination or that may be triggered by the vaccine.
Patient Variables
Pediatrics
Immunizations are an integral part of the primary health care of children. Recent research concluded that childhood vaccines, with the exception of the vaccine for measles, mumps, and rubella, were not linked to an increased risk of immune thrombocytopenic purpura among young children. However, the hepatitis A vaccine was associated with an increased ITP risk among 7- to 17-year-olds, while the chickenpox and TdaP vaccines were tied to the disorder among 11- to 17-year-olds.
Children who were exposed to more perfluorinated compounds have been found to be less likely to respond to routine vaccines, according to a study in the Journal of the American Medical Association. Researchers found that a doubling in maternal blood levels of perfluorooctane sulfonic acid was tied to a 39% decline in diphtheria antibody concentration in children at age 5.
Patient Education
With most vaccines, the arm in which the vaccine is given may become sore; this can be treated with a warm compress. Some patients develop generalized flu-like symptoms. For any feeling of malaise or flu, the patient often is instructed to take acetaminophen. Most symptoms are mild and self-limited. Reactions that should be reported to the health care provider are discussed separately.
Notify patients, parents, or guardians of the risks and benefits of each individual vaccine, and give them the standardized information pamphlets.