physicochemical barriers, e.g. intact skin and mucous membrane, low stomach pH, antibacterial agents (lysozyme) in skin and tear secretions,
 macrophages and dendritic cells, particularly in lungs, liver, lymph nodes and spleen, phagocytose pathogenic material and produce antigen fragments (short peptides of approximately 8–25 amino acids) from the pathogenic material, which they then display on their surfaces. The cells are then described as antigen-presenting cells (APCs) and are necessary for the presentation of the antigen to T-lymphocytes and the triggering of the adaptive immune system (see below),
macrophages and dendritic cells, particularly in lungs, liver, lymph nodes and spleen, phagocytose pathogenic material and produce antigen fragments (short peptides of approximately 8–25 amino acids) from the pathogenic material, which they then display on their surfaces. The cells are then described as antigen-presenting cells (APCs) and are necessary for the presentation of the antigen to T-lymphocytes and the triggering of the adaptive immune system (see below), attraction of immune cells to sites of inflammation by substances released from cells such as cytokines,
attraction of immune cells to sites of inflammation by substances released from cells such as cytokines, phagocytosis of bacteria and parasites by granulocytes, including neutrophils, monocytes and macrophages,
phagocytosis of bacteria and parasites by granulocytes, including neutrophils, monocytes and macrophages, binding of antigens to IgE antibody on mast cells and basophils and the subsequent release of inflammatory mediators from the cell,
binding of antigens to IgE antibody on mast cells and basophils and the subsequent release of inflammatory mediators from the cell,The innate immune system may be an adequate defence to deal with many pathogens but, unlike adaptive immunity, long-term specific immune protection following initial exposure to a pathogen does not occur.
Adaptive immunity
Adaptive immunity is superimposed upon the innate mechanisms. It differs from innate immunity in that it is slower to respond, offers long-term specific protection and has exquisite specificity in recognising individual non-self molecules. Adaptive immunity has two basic complementary and interacting mechanisms: cell-mediated immunity and humoral immunity (Figs 38.1 and 38.2).
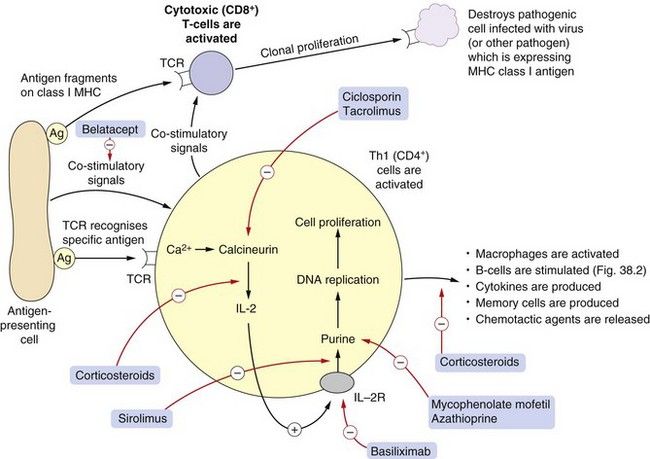
Fig. 38.1 Aspects of cell-mediated immunity.
This shows in simplified form some steps in T-cell activation following antigen presentation to the T-cell receptor (TCR). Pathogenic antigens are presented by antigen-presenting cells to the uncommitted CD4+ lymphocyte which carries the specific receptor to the antigen, in association with major histocompatibility complex (MHC) class I and co-stimulatory molecules. Under the influence of interleukin-2 (IL-2), Th1 cells undergo clonal proliferation and play a variety of roles in cell-mediated immunity, including activation of macrophages and other cells. Antigens can also be presented to CD8+ lymphocytes, which mature into cytotoxic T-cells. Drugs used as immunosuppressants (red arrows) act at the sites shown. Corticosteroids act at many sites (see also Fig. 38.2 and Ch. 44). Ag, antigen; IL-2R, interleukin-2 receptor; Th, T-helper cell.
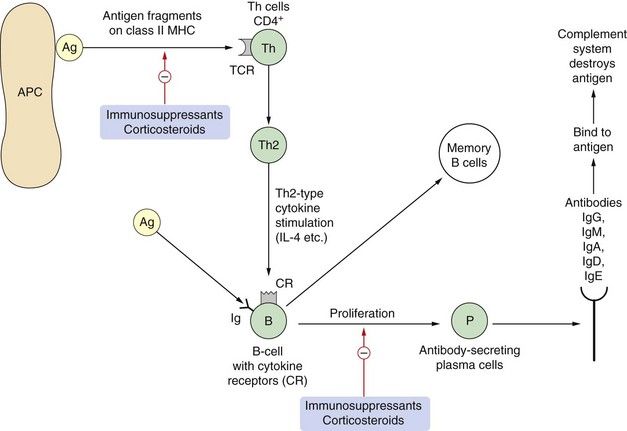
Fig. 38.2 Aspects of humoral immunity.
Adaptive immunity can result in production of antibodies (humoral response) or a cell-mediated response (Fig. 38.1). Antigens on bacteria or bacterial toxins bind to immunoglobulins on B cells. Before proliferation and antibody production can occur, the B-cell also has to be stimulated by activated T-cell cytokine production, usually of the Th2 type. Antigen fragments can be presented with major histocompatibility complex (MHC) class II molecules to T-cells via a T-cell receptor (TCR) that recognises the antigen. T-cells undergo clonal proliferation and produce cytokines that stimulate B-cells to produce humoral antibodies (IgG, IgM, IgA, IgD and IgE). In atopic individuals, the T-cells are tipped towards the Th2 type and produce interleukins IL-4, IL-5, IL-10 and transforming growth factor β (TGFβ), which induce the B-cells to produce IgE. Ag, antigen; APC, antigen-presenting cell; CR, cytokine receptor; P, plasma cell; Th, T-helper cell.
Essential components of the adaptive system are the two populations of lymphocytes:
 T-lymphocytes are produced in the bone marrow and migrate to the thymus, where they mature, express receptors for antigens and interact with immunogenic self peptides. T-cells are selected in the thymus for low or high avidity for self peptides, and those showing high avidity are destroyed. The surviving T-cells retain the potential to cross-react with multiple foreign non-self antigens but not self molecules.
T-lymphocytes are produced in the bone marrow and migrate to the thymus, where they mature, express receptors for antigens and interact with immunogenic self peptides. T-cells are selected in the thymus for low or high avidity for self peptides, and those showing high avidity are destroyed. The surviving T-cells retain the potential to cross-react with multiple foreign non-self antigens but not self molecules.T-cells and B-cells are coated with vast numbers of proteins which act as receptors or as ligands for other receptors. These proteins can be defined by antibody typing (immunophenotyping) and are given cluster of differentiation (CD) numbers such as CD4, CD8, etc. When T-cells leave the thymus they are considered naïve or uncommitted since they have not yet been exposed to the non-self antigens. At this stage, naïve T-cells consist of two major populations, known as helper (Th; CD4+) and cytotoxic or killer (Tc; CD8+) T-cells (Fig. 38.1). Immunogenic peptides are presented to T-cells within the cleft of major histocompatibility complex (MHC) class II molecules on the surface of APCs from the innate immune system. The CD4+ (Th) cells have surface receptors with a high affinity for class II MHC which binds antigenic peptides on APCs. The CD8+ (Tc) cells have an affinity for class I MHC, which displays specific antigens on the surface of tumour cells or infected host cells.
The CD4+ Th-cell is activated if its receptors recognise and bind avidly to an antigen, but only if the antigen is processed and presented on an APC. This triggers a series of complex activation pathways which prepare the Th-cell for its immune role. Acivated Th-cells secrete the T-cell growth factor interleukin-2 (IL-2), which acts in an autocrine fashion on the Th-cells and causes them to proliferate. The Th-cells are then committed to become a type 1 Th-cell (Th1) or a type 2 Th-cell (Th2). The pattern of differentiation may be determined by the type of antigen or possibly the concentration of antigen presented to the Th-cell. Differentiated Th-cells orchestrate the immune response by secretion of specific cytokines (Figs 38.1 and 38.2). Th1-cells interact with macrophages to enhance their phagocytic activity and stimulate the proliferation of cytotoxic Tc-cells. Th2-cells stimulate B-cells to grow and divide, and activate humoral immune responses. The details of these responses are not well understood, and the existence of other Th-cell populations (such as Th3 and Th17) adds to the complexity of this cell family. The activities of Th-cells and APCs are modulated by regulatory T-cells (Treg).
Co-stimulatory signals involving cell surface CD proteins are important processes in immune cell function. For example, CD40 protein on APCs interacts with Th-cell proteins and has a co-stimulatory role in APC activation. The CD80 and CD86 proteins on APCs interact with CD28 proteins on Th- and Tc-cells to co-activate them together with Th-cell cytokines. The expression of CD20 protein on B-cells enables an optimum immune response against T-cell-independent antigens (antigens that elicit a full humoral immune response without participation of T-cell cytokines).
Immature B-cells can bind antigen with the cooperation of T-cells, but on subsequent exposure antigen binds directly to immunoglobulins on the B-cell (Fig. 38.2)
Cell-mediated immunity
Cell-mediated immunity is largely T-cell-driven, utilising Th1 (CD4+) and cytotoxic T-cell (CD8+) subtypes, and is involved in responses to viral infection, graft rejection, chronic inflammation and tumour immunity. Figure 38.1 shows schematically the basic processes occurring in cell-mediated immunity. T-cells that possess the receptor to the antigen of the invasive pathogen that is presented on APCs are stimulated to express IL-2 and the IL-2 receptor. For clarity, the many co-stimulatory processes that are described in the text above are not shown in the figure.
Stimulation of the IL-2 receptor induces the Th-cell to:
Cytotoxic T-cells that recognise the foreign antigen presented on MHC type I on APCs are activated to proliferate and attack pathogens expressing the antigen. When cytotoxic cells bind to an antigen on a pathogenic cell they release a variety of proteases or lysins to destroy the cell.
Humoral immunity
Figure 38.2 illustrates the basic processes in humoral immunity. The foreign antigen is recognised by immunoglobulin (Ig) molecules or specific receptors to that antigen on the surface of a specific clone of B-cells. The presence of nearby Th2-cells that have been activated to secrete IL-5 and IL-10 is required for initial antigen recognition by B-cells. The secreted interleukins cause B-cell clonal proliferation, and convert B-cells into active plasma cells that can secrete antibodies of the IgG, IgM, IgA, IgD and IgE classes which bind to and destroy pathogenic antigens.
On encountering an antigen, the primary immune response consists of IgM, replaced later by IgG. B-cells that are primed to produce specific antibodies survive as memory B-cells. On a further encounter with the antigen, the secondary immune response occurs more rapidly and consists of large amounts of IgG produced by plasma cells derived from reactivation of memory B-cells.
Unwanted immune reactions
The processes of inflammation and immunity described above are essential to protect the host against pathogens and other damage, but excessive, inappropriately prolonged or misdirected immune responses can cause disease, including hypersensitivity reactions, graft rejection and autoimmune diseases.
It is not always easy to decide whether predominantly Th1- or Th2-mediated immune responses are involved in a particular disease; in part this is due to the fact that Th2 cytokines can inhibit Th1-cell functions. Th1-mediated immune responses are significantly involved in rheumatoid arthritis (Ch. 30) and in the formation of atheroma (Ch. 48). Th2-mediated responses are important in mild to moderate asthma but with increasing participation of Th1-mediated responses in severe asthma (Ch. 12).
Hypersensitivity reactions
Hypersensitivity reactions were classified by Gell and Coombs in the late 1950s.
Type 1 (acute, immediate): This category includes hay fever and acute asthma. IgE molecules on the surface of mast cells and basophils are crosslinked by harmless antigens (allergens such as pollens or house dust mites), leading to the synthesis and/or release of inflammatory mediators. These include cysteinyl-leukotrienes, prostaglandins, histamine, platelet-activating factor, proteases and cytokines.
Type 2 (cytotoxic): Cell surface antigens, including microbial proteins and drug molecules haptenised onto cell surfaces, are recognised and bound by IgG and IgM antibodies (opsonisation), leading to activation of complement (classic pathway) and cytolysis of the target cell. Examples include destruction of red cells after incompatible blood transfusion, and haemolytic anaemia caused by binding of some drugs to host cells (see Ch. 53).
Type 3 (complex-mediated): Soluble antigens react with excess circulating antibodies to form complexes that precipitate in small blood vessels, causing vasculitis and organ damage. The various forms of extrinsic allergic alveolitis, caused by exposure to animal or vegetable dusts, are systemic type 3 reactions, while the Arthus reaction is a local response to an injected antigen (e.g. non-human insulins).
Type 4 (cell-mediated, delayed-type hypersensitivity): Inappropriate regulation of cell-mediated immunity may cause damaging chronic inflammation, leading to fibrosis and granuloma formation. Cell-mediated immunity misdirected against harmless foreign proteins (allergens) can lead to chronic allergic inflammation (such as occurs in eczema), or cause contact sensitivity in the skin to haptenising metals and chemicals. In allergy, Th2-cells secrete cytokines, including IL-4, IL-5 and IL-13, which promote eosinophilic inflammation and overproduction of IgE by B-cells.
Transplant rejection
In blood transfusion, rejection usually occurs because non-self antigens on the transfused red blood cells (ABO system) trigger a type 2 hypersensitivity reaction in the recipient. In immunodeficient people, transfused T-cells react against recipient antigens (graft-versus-host reactions).
For organ transplants, hyperacute rejection can occur if there is ABO incompatibility, or host-versus-graft reactions can arise later with foreign MHC molecules (human leucocyte antigens, HLA). The latter can be reduced by HLA tissue typing to increase the chance of selecting a graft that is compatible with the host tissues. This will reduce the rate of tissue destruction but not prevent chronic rejection. The immune response and its place in the rejection of a transplanted organ are complex. The antigens on the graft are recognised as foreign, and the cascaded responses outlined in Figures 38.1 and 38.2 occur, with increased production of B-cells, cytotoxic T-cells and monocyte/macrophages. Graft destruction occurs from antibody production against the graft, lysis of graft cells and delayed hypersensitivity responses. Rejection can be immediate (days), acute (weeks) or chronic (years).
Autoimmunity
Normally the immune system is tolerant of self antigens. T-cells in the thymus that express receptors with high avidity for self peptides are normally destroyed in a process known as negative selection (see above). If this self-tolerance breaks down, autoimmune disorders result. Numerous mechanisms can trigger autoimmune diseases, including viral infection of host cells, binding of drug molecules to host cells (e.g. penicillin), sharing of antigens between host cells and microbes and sequestration of antigens liberated by cell damage. Examples of autoimmune disease include haemolytic anaemia, type 1 diabetes mellitus, Addison’s disease, rheumatoid arthritis, myasthenia gravis, systemic lupus erythematosus and Graves’ disease.
Immunosuppressant drugs
The immune system presents a large number of potential molecular targets for therapeutic intervention. Drugs currently used to suppress the immune response tend to be non-specific immunosuppressants with a range of unwanted effects. Immunosuppressant drugs are widely used in many diseases; examples include rheumatoid arthritis, psoriasis and inflammatory bowel disease. Their benefit is achieved both through modulation of the immune system and, in some cases, through their anti-inflammatory properties. Drug such as methotrexate and cyclophosphamide which are used for their cytotoxic actions in cancer chemotherapy (Ch. 52), are also used for their immunosuppressant properties in various disease states. Methotrexate and cyclophosphamide have immunosuppressant properties at doses much lower than those required to treat malignancy (Ch. 52). Corticosteroids (e.g. dexamethasone and prednisone; Ch. 44) are highly effective anti-inflammatory drugs that can be used systemically to suppress type 4 hypersensitivity reactions, autoimmune diseases and graft rejection. They are also used topically for inflammatory skin disease (Ch. 49), inflammatory bowel disease (Ch. 34) and allergic rhinitis (Ch. 39), and by inhalation for asthma (e.g. beclometasone, fluticasone; Ch. 12).
Co-stimulatory molecules on immune cells have recently been targeted in the development of drugs for the treatment of autoimmune disease: belatacept is an antibody that binds to CD80 and CD86 proteins on APCs and rituximab is an antibody that binds to CD20 on B-cells, thus modulating the destructive immune responsiveness against self molecules in disease (Ch. 30, Fig. 30.1).
As an alternative to immunosuppression, inflammatory mediators released during immune reactions can be blocked by antagonists at their receptors on target cells, or by inhibiting their synthesis. This is a useful strategy for management of allergic disorders and some inflammatory conditions. Anti-mediator drugs include histamine H1 receptor antagonists (antihistamines), cysteinyl-leukotriene receptor antagonists (LTRAs) and cyclo-oxygenase inhibitors (non-steroidal anti-inflammatory drugs, NSAIDs). Antihistamines (e.g. loratadine and cetirizine) are used in the control of hay fever and other allergic disorders (Ch. 39), while oral LTRAs (e.g. montelukast and zafirlukast) are used in asthma (Ch. 12). Oral NSAIDs block the synthesis of prostaglandins and are used in inflammatory soft-tissue disorders and arthritis (Chs 29 and 30).
Calcineurin inhibitors
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree











