The Hypothalamus and Pituitary Gland
GENERAL PRINCIPLES OF ENDOCRINE DIAGNOSIS
A hormone can be defined as a substance secreted by an endocrine gland that is transported in the blood, thereby regulating the function of another tissue(s). Certain hormones, such as growth hormone (GH, secreted from the anterior pituitary gland), thyroxine (T4, from the thyroid gland) and insulin (from the pancreatic islet cells), influence tissue metabolism directly. Conversely, trophic hormones from the pituitary gland stimulate target endocrine glands to synthesize and secrete further hormones, which in turn partly control trophic hormone release, usually by negative feedback inhibition. For example, hypercalcaemia inhibits the secretion of parathyroid hormone (PTH), and elevation of plasma T4 concentration inhibits the secretion of thyroid-stimulating hormone (TSH).
Endocrine glands may secrete excessive or deficient amounts of hormone. Abnormalities of target glands may be primary or secondary to dysfunction of the controlling mechanism, usually located in the hypothalamus or anterior pituitary gland.
Hormone secretion may vary predictably over a 24-h (circadian) or longer period. It may be episodic or may respond predictably to physiological stimuli such as stress. Simultaneous measurement of both the trophic hormones and their controlling factors, whether hormones or metabolic products, may be more informative than the measurement of either alone. An important endocrine principle is that an apparently ‘normal’ hormone result should be interpreted in the context of the associated hormone axis, for example a plasma PTH concentration within the reference range may be abnormal if the plasma calcium concentration is elevated.
It is also important to know about the assay’s performance, as sometimes heterophilic interfering antibodies may cross-react with various hormones, as can certain immunoglobulins, for example macroprolactin (see Chapter 9, Hyperprolactinaemia).
If the results of preliminary tests are definitely abnormal, this may be primary or secondary to a disorder of one of the controlling mechanisms. Should the results be equivocal when considered together with the clinical findings, so-called ‘dynamic’ tests should be carried out. In such tests the response of the gland or the feedback mechanism is assessed after stimulation or suppression by the administration of exogenous hormone.
Suppression tests are used mainly for the differential diagnosis of excessive hormone secretion. The substance (or an analogue) that normally suppresses secretion by negative feedback is administered and the response is measured. Failure to suppress implies that secretion is not under normal feedback control (autonomous secretion).
Stimulation tests are used mainly for the differential diagnosis of deficient hormone secretion. The trophic hormone that normally stimulates secretion is administered and the response is measured. A normal response excludes an abnormality of the target gland, whereas failure to respond confirms it.
Disorders of the pituitary gland and hypothalamus are discussed in this chapter. Diseases of the target endocrine organs, the adrenal cortex, gonads and thyroid gland, are considered in Chapters 8, 9 and 11 respectively. The parathyroid glands and endocrine pancreas are discussed in Chapters 6 and 12 respectively.
HYPOTHALAMUS AND PITUITARY GLAND
The anterior and posterior lobes of the pituitary gland are developmentally and functionally distinct; both depend on hormones synthesized in the hypothalamus for normal function. The hypothalamus also has extensive neural connections with the rest of the brain, and stress and some psychological disorders affect the secretion of pituitary hormones and of the hormones from other endocrine glands; see also Chapter 9.
Control of posterior pituitary hormones
Two structurally similar peptide hormones, antidiuretic
hormone (ADH) – also called vasopressin or arginine vasopressin (AVP) – and oxytocin, are synthesized in the hypothalamus and transported down the nerve fibres of the pituitary stalk attached to specific carrier proteins – neurophysins. The hormones are stored in the posterior pituitary gland and are released independently of each other into the bloodstream under hypothalamic control, together with neurophysin. Neurophysin has no apparent biological function and is rapidly cleared from plasma.
hormone (ADH) – also called vasopressin or arginine vasopressin (AVP) – and oxytocin, are synthesized in the hypothalamus and transported down the nerve fibres of the pituitary stalk attached to specific carrier proteins – neurophysins. The hormones are stored in the posterior pituitary gland and are released independently of each other into the bloodstream under hypothalamic control, together with neurophysin. Neurophysin has no apparent biological function and is rapidly cleared from plasma.
Antidiuretic hormone (arginine vasopressin) is mainly synthesized in the supraoptic nuclei of the hypothalamus and enhances water reabsorption from the collecting ducts in the kidneys (see Chapters 2 and 3).
Oxytocin is synthesized in the paraventricular nuclei of the hypothalamus. It controls the ejection of milk from the lactating breast and may have a role in initiating uterine contractions, although normal labour can proceed in its absence. It may be used therapeutically to induce labour.
Anterior pituitary hormones
There is no direct neural connection between the hypothalamus and the anterior pituitary gland. The hypothalamus synthesizes small molecules (regulating hormones or factors) that are carried to the cells of the anterior pituitary lobe by the hypothalamic portal system. This network of capillary loops in the median eminence forms veins, which, after passing down the pituitary stalk, divide into a second capillary network in the anterior pituitary gland, from where hypothalamic hormones stimulate or inhibit pituitary hormone secretion into the systemic circulation.
The cells of the anterior pituitary lobe can be classified simply by their staining reactions as acidophils, basophils or chromophobes. Immunohistochemistry can identify specific hormone-secreting cells.
Acidophils are of two cell types:
lactotrophs, which secrete prolactin,
somatotrophs, which secrete GH (somatotrophin).
These hormones, which are simple polypeptides with similar amino acid sequences, mainly affect peripheral tissues directly. Stimulation and inhibition of secretion via the hypothalamus is influenced by neural stimuli.
Basophils secrete hormones that affect other endocrine glands. The hypothalamic control is mainly stimulatory. There are three cell types:
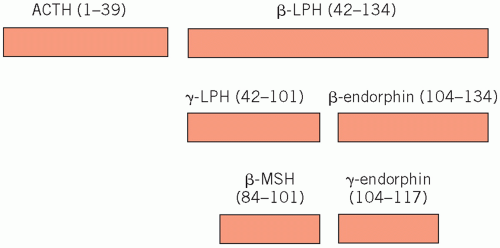
Corticotrophs synthesize a large polypeptide (pro-opiomelanocortin), which is a precursor of both adrenocorticotrophic hormone (ACTH; corticotrophin) and β-lipotrophin (Fig. 7.1). Secretion of these hormones occurs in parallel.
Adrenocorticotrophic hormone stimulates the synthesis and secretion of steroids, other than aldosterone, from the adrenal cortex and maintains adrenal cortical growth. Part of the molecule has melanocyte-stimulating activity, and high circulating concentrations of ACTH are often associated with pigmentation.
β-Lipotrophin is inactive until rapidly converted to endorphins. These are neurotransmitters which, because they have opiate-like effects, help control pain.
Gonadotrophs secrete the gonadotrophins, folliclestimulating hormone (FSH) and luteinizing hormone (LH), which act on the gonads.
Thyrotrophs secrete TSH (thyrotrophin), which acts on the thyroid gland.
These hormones are structurally similar glycoproteins consisting of two subunits, α and β. The α-subunit is common to all three hormones; the β-subunit is important for receptor recognition and therefore in specific biological activity.
Chromophobes, once thought to be inactive, do contain secretory granules. Chromophobe adenomas often secrete hormones, particularly prolactin.
Control of anterior pituitary hormone secretion
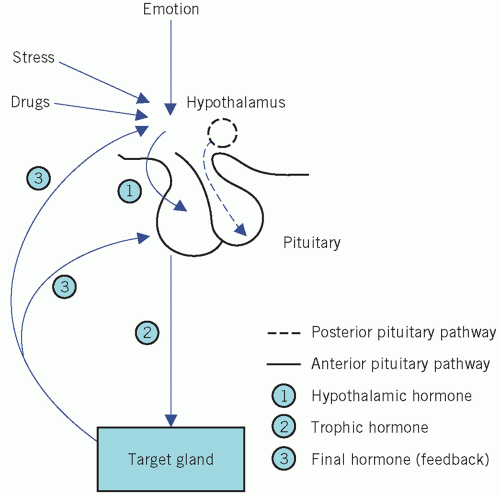
Neural and feedback controls are the two most important physiological factors influencing the secretion of the anterior pituitary hormones (Fig. 7.2).
Extrahypothalamic neural stimuli modify, and at times over-ride, other control mechanisms. Physical or emotional stress and mental illness may give similar findings to, and even precipitate, endocrine disease. The stress caused by insulin-induced hypoglycaemia is used to test anterior pituitary function. Stress may also stimulate the secretion of ADH from the posterior pituitary.
Feedback control is mediated by the concentrations of circulating target-cell hormones; a rising concentration usually suppresses trophic hormone secretion. This negative feedback may directly suppress hypothalamic hormone secretion or may modify its effect on pituitary cells (long feedback loop). The secretion of hypothalamic hormones may also be suppressed by rising concentrations of pituitary hormone in a short feedback loop.
Inherent rhythms: hypothalamic, and consequently pituitary, hormones are released intermittently, either in pulses or in a regular circadian rhythm. Disturbances of such rhythms may be of diagnostic value. This subject is considered further in the relevant sections.
Drugs may also stimulate or block the action of neurotransmitters, such as catecholamines, acetylcholine and serotonin, and influence the secretion of hypothalamic, and consequently pituitary, hormones. The following are some examples.
Certain neuroleptic drugs, such as chlorpromazine and haloperidol, interfere with the action of dopamine. This results in reduced GH secretion (reduced effect of releasing factor) and increased prolactin secretion (reduced inhibition).
Bromocriptine, which has a dopamine-like action, and levodopa, which is converted to dopamine, have the opposite effect in normal subjects. Bromocriptine causes a paradoxical suppression of excessive GH secretion in acromegalics; the reason for this anomalous response is unknown.
All these effects have been used in both the diagnosis and treatment of hypothalamic-pituitary disorders; they are discussed in later sections.
Evaluation of anterior pituitary function
The interpretation of the results of basal pituitary hormone assays is often difficult. Low plasma concentrations are not necessarily abnormal, and plasma concentrations within the reference range do not exclude pituitary disease. The diagnosis of suspected hypopituitarism is best excluded by the direct measurement of pituitary hormones after stimulation or by demonstrating target gland hyposecretion after the administration of the relevant trophic hormone. However, prolonged hypopituitarism may result in secondary failure of the target gland with diminished response to stimulation.
Laboratory tests establish only the presence or absence of hypopituitarism, and the cause must be sought by other clinical means such as radiological imaging (see also Chapter 9).
Hypothalamus or pituitary dysfunction?
It may be difficult to distinguish between hypothalamic and pituitary causes of pituitary hormone deficiency or, more correctly, between deficient releasing factor and a primary deficiency of pituitary hormone secretion. Isolated hormone deficiencies are more likely to be of hypothalamic than of pituitary origin. The coexistence of diabetes insipidus suggests a hypothalamic disorder.
Some biochemical investigations evaluate both hypothalamic and pituitary function and some only the latter, although it may be possible to distinguish the anatomical site of the lesion. For example, the TSH response to thyrotrophin-releasing hormone (TRH) may differ in hypothalamic and pituitary causes of secondary hypothyroidism (see Chapter 11). In cases of hypogonadism due to gonadotrophin deficiency, differentiation on the basis of the response to
gonadotrophin-releasing hormone (GnRH) is less clear cut (see Chapter 9).
gonadotrophin-releasing hormone (GnRH) is less clear cut (see Chapter 9).
DISORDERS OF ANTERIOR PITUITARY HORMONE SECRETION
The main clinical syndromes associated with excessive or deficient anterior pituitary hormone secretion are shown in Table 7.1. Excessive secretion usually involves a single hormone, but deficiencies are often multiple. However, many pituitary tumours are non-secretory and may present clinically with eye signs or headaches.
Growth hormone
Growth hormone secretion from the anterior pituitary gland is mainly controlled by hypothalamic GH-releasing hormone (GHRH). After synthesis by the hypothalamus, this is transported via the hypothalamic portal system to the somatotrophs of the anterior pituitary. Secretion of GHRH, and therefore of GH, is pulsatile, occurring about seven or eight times a day, usually associated with:
exercise,
onset of deep sleep,
in response to the falling plasma glucose concentration about an hour after meals.
At other times, plasma concentrations are usually very low or undetectable, especially in children.
Growth hormone release is inhibited in a negative feedback pathway by another hypothalamic hormone, somatostatin (GH-release inhibiting hormone). Somatostatin is found not only in the hypothalamus and elsewhere in the brain, but also in the gastrointestinal tract and pancreatic islet cells, where it inhibits the secretion of many gastrointestinal hormones. Insulinlike growth factor 1 (IGF-1) acts by feedback to inhibit GHRH action.
Table 7.1 Disorders associated with primary abnormalities of anterior pituitary hormone secretion | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Growth hormone secretion may be stimulated by:
stress, one cause of which is hypoglycaemia,
glucagon,
some amino acids, for example arginine,
drugs such as levodopa and clonidine.
All these stimuli have been used to assess GH secretory capacity, which may also be impaired in obese patients, in hypothyroidism and hypogonadism, in some cases of Cushing’s syndrome and in patients receiving large doses of steroids.
Actions of growth hormone
The main function of GH is to promote growth. Its action is primarily mediated by IGFs, polypeptides that are synthesized in many tissues, where they act locally. Plasma concentrations of one of these, IGF-1 (also known as somatomedin C), correlate with GH secretion.
Carbohydrate metabolism is affected by GH: GH antagonizes the insulin-mediated cell uptake of glucose, and excess secretion may produce glucose intolerance.
Fat metabolism is stimulated by GH: lipolysis is stimulated, with a consequent increase in the concentration of circulating free fatty acids. Free fatty acid antagonizes insulin release and action. Growth hormone enhances protein synthesis, in conjunction with insulin, to stimulate amino acid uptake by cells.
The production of IGF-1 is also influenced by other factors, the most important of which is nutritional status. In undernutrition, plasma concentrations are low, whereas GH concentrations are elevated, suggesting that plasma IGF-1 may influence GH secretion by negative feedback. Other factors, such as adequate nutrition and T4, are also needed for normal growth. The growth spurt during puberty may be enhanced by androgens.
Growth hormone excess: gigantism and acromegaly
Growth hormone excess causes gigantism during childhood and acromegaly in adults.
Most patients with GH excess have acidophil adenomas of the anterior pituitary gland, which may be secondary to excessive hypothalamic stimulation. Rarely, malignant tumours may release GH or GHRH.
Acromegaly is sometimes one of the manifestations of multiple endocrine neoplasia (MEN).
Acromegaly is sometimes one of the manifestations of multiple endocrine neoplasia (MEN).
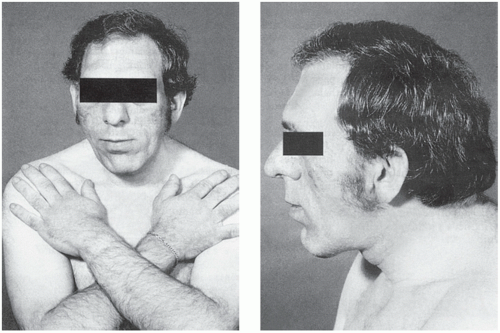
The clinical manifestations of GH excess depend on whether the condition develops before or after fusion of the bony epiphyses. Gigantism is caused by excess GH secretion in childhood before fusion of the epiphyseal plates, which may be delayed by accompanying hypogonadism. Heights of up to about 2 metres may be reached. Acromegalic features may develop after bony fusion, but these patients may die in early adult life from infection or cardiac failure or as a consequence of progressive pituitary tumour growth. The features of acromegaly may include the following (Fig. 7.3):
An increase in the bulk of bone and soft tissues with enlargement of, for example, the hands, tongue, jaw and heart. Changes in facial appearance are often marked, due to the increasing size of the jaw and sinuses; the gradual coarsening of the features may pass unnoticed for many years. Thyroid gland enlargement may be clinically detectable, but the patient is usually euthyroid.
Excessive hair growth, hyperhidrosis and sebaceous gland secretion are common.
Menstrual disturbances are common in females.
Impaired glucose tolerance is present in about 25 per cent of patients, about half of whom develop symptomatic diabetes mellitus. In most cases the pancreas can secrete enough insulin to overcome the antagonistic effect of GH.
There is a predisposition to multiple pre-malignant colon polyposis and hypertension.
Hyperphosphataemia, hypercalcaemia and hypertriglyceridaemia may also be present.
Many of these features are due to the action of IGF-1, which acts as a general growth factor.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree