(1)
University of South Australia, Adelaide, Australia
Abstract
This chapter introduces five concepts central to this book. The opportunity cost of a strategy in an institutional setting is identified when the decision maker values all states of the world that could emerge under different allocations of resources, not just the alternative options available to her or him. Price-effectiveness analysis is a method of assessing the decision to reimburse a new drug by testing the relationship between the incremental price-effectiveness ratio (IPER) of the new drug and the population’s health. The strategy of reimbursement comprises the actions of adoption and financing. The health shadow price, β c is the IPER of the health effects gained by the target patients as a consequence of the strategy of reimbursing (adopting and financing) the new drug with clinical innovation and additional financial cost such that the funder is indifferent between the strategy of reimbursement and the best alternative strategy available to the funder using the same financial resources. The economic value of clinical innovation (EVCI) is the gross clinical benefit of the new drug, constrained twice: by the clinical opportunity cost (the best alternative therapy to the new drug) and the economic opportunity cost (the best alternative use of resources). The health shadow price β c and EVCI are derived for the case of an economically efficient fixed budget with no failure in the market for health inputs.
6.1 The Reimburser’s Problem
The Reimburser understands that the choice of the decision threshold for a new drug is a complex one, both technically and politically. She also understands it is related to the idea of the appropriate valuation in a market context of the objectively determined therapeutic significance of a new drug. After discussions with her Health Economic Adviser about clinical innovation and the shadow price she decides that what she needs is an average shadow price for the health effects from the new drug, which is expressed as a decision threshold and is calculated with reference to the best alternative use of the incremental financial cost of the new drug, ΔC (Chap. 5). A firm can then use this information as a signal of the maximum acceptable IPER of the new drug. But how should she arrive at such a measure? The Reimburser asks her Health Economic Adviser:
Is there a shadow price for the health effects of a new drug that:
Is based on the opportunity cost of the best alternative way to produce health effects;
Can be used as a decision threshold IPER for a new drug; and
Is sensitive to the economic context?
6.2 The Path to the Health Shadow Price
The Health Economic Adviser provides the Reimburser with the path that he used to develop the idea of the objective value of the health effects of a new drug, the health shadow price. This path is illustrated in Fig 6.1. The Reimburser works her way along this path, starting with the question of the appropriate value of “the objectively demonstrated therapeutic significance of a pharmaceutical”. The Reimburser recognises that this is about the value of the clinical innovation in the context of an economic transaction, where the patent holder (the firm) has market power. However, as a large monopsonist purchaser, she also has some market power. In this situation she can choose to value the new drug using either an economic concept (opportunity cost) or a lay concept (value for money) that does not recognise the economic context. The former is preferable to the latter in this context.
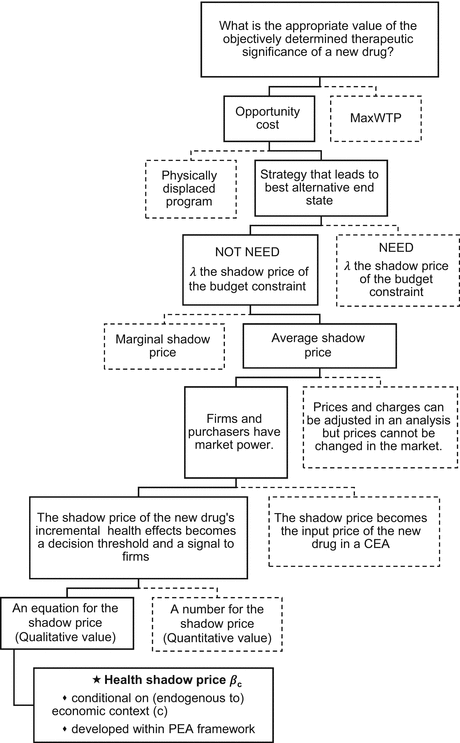

Fig. 6.1
The path to a health shadow price
The opportunity cost of reimbursing a new drug is not what is physically displaced to finance the new drug; that is an operational issue. The opportunity cost is the best alternative strategy to reimbursement. Furthermore, as a member of an institution, the Reimburser does not need to focus only on alternative strategies physically available to her. According to Buchanan (2008), in an institutional setting, the use of the term “opportunity cost to the decision” does not necessarily imply that the decision maker is physically choosing between these two strategies and their corresponding end-state alternatives. Instead, it means that the decision maker is valuing all states of the world that could emerge under different allocations of resources, in this case, the resources with a financial value of ΔC. This definition overcomes, at some level at least, the possible failure of the institution to include these alternatives in the physical choice set; in particular, unpatented services such as workforce strategies, respite care and training of health workers.1
The Reimburser does not need to know the shadow price of the budget constraint, λ, in order to understand the opportunity cost of reimbursement. In fact it is very likely that this cannot be defined, given the current levels of inefficiency in the health budget. This shadow price should reference the average shadow price of the best alternative input (Kim and Cho 1988). In the context of the reimbursement process, the common reference point across alternative inputs is the amount ΔC, the incremental cost of the new drug at the offer price, the IPER. The opportunity cost of reimbursement is the maximum health effects foregone by allocating ΔC to the reimbursement of the new drug rather than alternative strategies.
Firms, and in some cases the purchaser, have market power; they are price makers not price takers. The patent-owning firm can—and must—select a price for the new drug, unlike the case of a perfectly competitive market for a given drug, where the firm must accept the market price. The Reimburser can use the decision threshold to provide signals to firms, and change this signal if she chooses, as the competitiveness of the market for health inputs changes. The issue of interest is the Reimburser’s choice of the decision threshold. The principle suggested by Drummond et al. (2005) is to adjust the input price in a HTA/CEA to reflect its social opportunity cost.2 This method is not appropriate in this situation; the new drug price problem is about the Reimburser providing a signal about the market for health inputs and the firm using this signal to select an offer price. New drug reimbursement is not an extension of the problem of correcting a charge for an input in a CBA.
Finally, the Reimburser recognises that the initial choice is about the qualitative value (an equation) of the threshold, not the quantitative value.3 She notes that there appears to be more certainty regarding the quantitative value of NICE’s threshold than its qualitative value.4 She also recognises that only a qualitative value can be assessed in a theoretical context. Furthermore, a given qualitative value could provide a unique quantitative value for each decision. It is also important to accommodate the possibility that this threshold will be a function of a range of factors, including competition in the market, and hence is likely to vary over time and across decisions.
6.3 PEA, β c and the Economic Value of Clinical Innovation
In a discussion on the significance of alternative methods of determining the unit cost that should be used in an economic evaluation, when both charges and costs are available, Drummond et al. (2005) conclude that if study results are “relatively insensitive” to choices made about what to identify as the cost of an input then choice of methods only matters if, when studies are compared, differences in these methods are a driver of differences in the final ICER.5
PEA reframes how health economists understand the problem of a price for an input, when that input is patented. In PEA it is understood that even if the assessment resulting from a CEA is not sensitive to the way that the unit costs are derived (e.g., charges, or social opportunity cost), the firm’s choice of price is sensitive to this choice of method, if the patent holder of this input has market power. If the decision that results from a CEA is not sensitive to the method of costing, and hence price of one of the inputs, then this could be a signal to the owner of this input that the price can be increased without changing the assessment (purchase or not purchase) by the decision maker. That is, even if the drug continues to be assessed as “cost-effective” if the drug “price” is varied in the analysis, (and hence the decision by the purchaser does not vary), the price that a manufacturer will offer will vary as the signal to the firm (the decision threshold) varies.
Furthermore, the maximum acceptable price for the input can be inferred from the value assigned by the Reimburser to health effects via the decision threshold, (as discussed in Part 1 of the dung beetle story). The price assigned to an input either directly (via a unit cost in a HTA/CEA) or indirectly via a decision threshold matters because the patent-holding firm can respond to this signal in ways that impact on both consumer welfare and social welfare. The implications of this strategic context are discussed in detail in Chaps. 8 to 10. In this chapter, a method for determining a reference price, the health shadow price, is presented.
The PEA starting point is to reframe the problem of adjusting the price of an input in a HTA/CEA to reflect the input’s social opportunity cost6 as a problem of:
Developing a clear, objective and theoretically defensible method;
Identifying a qualitative value (equation) for the shadow price for the additional health effects of the new drug;
Referencing against the best alternative strategy; and
Applying this as a signal (decision threshold) in the reimbursement decision.
In this chapter I show that, when used together, the following five concepts provide a clear and objective method of introducing the shadow price into the reimbursement process.
Opportunity cost as the strategy that leads to the best end-state alternative. This strategy is not the physically displaced strategy (an operational issue) and not necessarily a physical option available to the Reimburser.
Reimbursement is a strategy comprising two actions: adoption of the new drug and financing of its additional costs.
PEA is a method whereby the relationship between the price of a new drug and the population’s health can be analysed.
The health shadow price,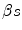 c , is the IPER7 of a new drug such that the Reimburser:
c , is the IPER7 of a new drug such that the Reimburser:
Is indifferent between: the strategy of reimbursing the new drug (adoption and financing); and the best alternative strategy for improving the population’s health (which could be improving efficiency).
Given: the economic context of the health budget (is it allocatively and technically efficient?); and the optimality with which the additional cost of adoption is financed from the health care budget.
The economic value of clinical innovation is the clinical value of innovation valued at β c . This is the appropriate value of the objectively determined therapeutic significance of a new drug, in the context of a market transaction.
6.3.1 The Problem
To illustrate the concepts and define the terminology we initially assume an economically efficient budget and perfectly competitive input markets (no market power). These assumptions are relaxed in Chaps. 7 and 8. The nominated strategy (reimbursement) is adoption financed by expansion of the budget (not by displacing existing services). Five parameters need to be defined to derive β c and hence the shadow price of the new drug in this situation:
1.
A maximand (the measure of effect);
2.
A nominated strategy and its corresponding effect(s);
3.
The constraints that define the set of strategies from which the best alternative strategy will be selected;
4.
The set of alternative strategies; and
5.
The best alternative strategy from this set.
A simple example of the decision to purchase a new drug and to finance its purchase with the expansion of a budget is used to illustrate these five elements.
1.
The effect or maximand is health, measured in QALYs.
2.
The nominated strategy (R) is reimbursement, which comprises the actions of adoption and financing.
(a)
Adoption: The adoption of a new Drug P is achieved by completely replacing Drug Q with Drug P for target patients. The incremental effect associated with adopting Drug P is ΔE P = 20 QALYs; the increase in health gains possible for the target patients following the adoption of the new Drug P compared with the best care they would otherwise receive (Drug Q).
(b)
Financing: The new drug has an additional financial cost of ΔC P = $1,000, which consists entirely of the additional cost of the drug (there are no other financial implications). The additional cost of this new drug is financed by the expansion of the existing health budget by $1,000. The firm’s offer price for the new drug, f, is expressed in terms of the IPER8:
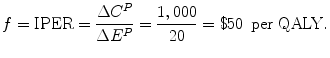
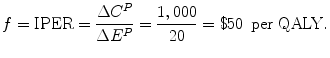
3.
Get Clinical Tree app for offline access

The constraints that define the set of alternative strategies (comprising adoption and financing actions) are:
(a)




The additional financial cost of Drug P ($1,000), that is, the alternative adoption action must have an additional financial cost of $1,000 and the financing action, in this case budget expansion, must raise this amount; and
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


