, Mohammed Al-Rubaie2, Stuart Walker3 and Sam Salek4
(1)
Regulatory Affairs Consultant Executive Directive, Kuwait Advancement for Conference and Exhibition Management, Kuwait, Kuwait
(2)
Ministry of Health, Directorate General of Pharmaceutical Affairs and Drug Control, Muscat, Oman
(3)
Founder of Centre for Innovation in Regulatory Science, London, UK
(4)
Department of Pharmacy School Life & Medical Sciences, University of Hertfordshire, Hatfield, UK
Introduction
The historical implementation of the central registration system was subject to several criticisms with challenges from both the pharmaceutical industry and government. The GCC-DR received 1,824 medicinal product applications out of which 1,165 (64 %) applications were approved during the 11 years from 1999 to 2010. The pharmaceutical industry was apprehensive about whether the GCC-DR system would be a hindrance to the timely approval of medicines in the region, whilst the government officials were concerned about losing sovereignty to the centralised authority (Hashan 2005). The collaborative efforts between the member countries however proved to be effective and substantiated the support of the GCC-DR system for each GCC country improving the regulatory approval processes and operation efficiencies at the national level.
Two members from each of the seven GCC countries represent the GCC Central Drug Registration (GCC-DR) Committee with the current procedure in force; two member countries are chosen to review a registration dossier. The selection of those countries is unbiased and purely based on alphabetical order, and all the GCC countries are equally responsible for evaluating the quality, safety and efficacy of medicines. Thus, all seven GCC countries consisting of Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, UAE and Yemen are provided with copies of the product registration dossier for their individual assessments. The member countries meet four to five times a year to discuss the product review reports issued by the reviewers from each country, and an approval decision is made on common agreement.
The only studies that have addressed the GCC region were those reported by Hashan in 2005 and later by Al-Essa in 2011. They reported that there are three common phases in the GCC regulatory review process: submission, evaluation and authorisation. The similarities and differences in the key milestones and activities in the GCC states made it possible to propose an improved process, which could underpin the degree of standardisation required for a strong regulatory body that facilitates an effective drug approval process for the region (Al-Essa 2011). These are the key components essential for the development of a strategic plan for strengthening drug regulations in the Gulf region. This chapter evaluates the experience and opinions of all seven Gulf states regulatory authorities with the GCC Central Registration Process.
The Views of Seven Gulf States About the Centralised Procedure
The regulatory authorities that are responsible for the regulation of pharmaceutical products in the seven Gulf states participated in the survey, and a 100 % response rating was achieved (Table 8.1). It was decided that Yemen should be part of this study because it has been an active member of the GCC-DR Committee since 2004.
Table 8.1
List of the seven participating authorities in the Gulf Cooperation Council (GCC) states
Position | Agency | Country |
|---|---|---|
Director, pharmacy and drug control | Ministry of Health | Bahrain |
Drug registration and release superintendent | Drug and Food Control – Ministry of Health | Kuwait |
Director general of pharmaceutical affairs and drug control | Direct General of Pharmaceutical Affairs & Drug Control – Ministry of Health | Oman |
Head of drug registration section | Drug Control Department – Supreme Council of Health | Qatar |
Executive director of licensing department – drug sector | Saudi Food & Drug Authority | Kingdom of Saudi Arabia (KSA) |
Head of registration and pricing department | Drug Control Department – Ministry of Health | UAE |
Member of the board council | Supreme board of drugs and medical appliances | Yemen |
Value of the Centralised Procedure (CP)
The complete responses from all GCC member states indicated that the Centralised Procedure (CP) had indeed contributed to an effective system for authorising medicinal products for the GCC. This was ascertained from their views that the GCC-DR achieved its main objective, by providing the best possible scientific opinion for the CP. They also agreed that the CP has appropriate guidelines for regulatory review in the GCC-DR and that the quality of the CP decision-making is satisfactory to good as compared to the national system.
The CP, which has been put in place since 1999, is clearly continuing to function efficiently, and six of the member countries indicated that the quality of the CP decision-making process is good. Only Saudi Arabia rated the quality of processes as satisfactory. This signifies that all GCC member countries support the Centralised Procedure in registering pharmaceutical products. With the strong support for the CP, four member countries (Kuwait, Saudi Arabia, UAE and Yemen) indicated that the CP should be extended to other products to include herbal products, health products and medical devices, whilst Bahrain, Oman, and Qatar reported that the CP should not be extended to other products (Fig. 8.1).
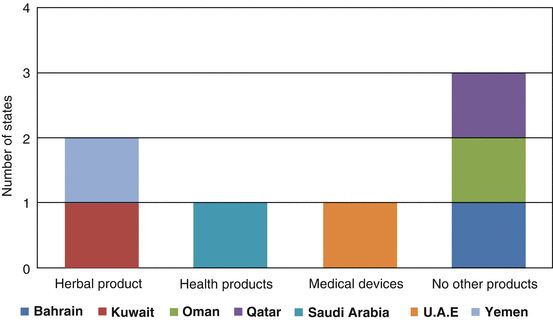

Fig. 8.1
The products which could potentially be registered under the Centralised Procedure
The suggested range for the approval timeline for products reviewed in the Centralised Procedure should be between 6 and 12 months as indicated by four of the member countries (Oman, Saudi Arabia, UAE, and Yemen), whilst Bahrain, Kuwait and Qatar indicated that it should be 3–6 months. The latest data available (2010) indicated that the median approval time for the Centralised Procedure was 9 months.
The long approval time could be the main obstacle in advocating the CP as the main route for registration of products in the region. As a result, five of the member countries (Bahrain, Kuwait, Oman, Saudi Arabia and UAE) agreed that the CP process could be shortened, although two (Yemen and Qatar) indicated otherwise (Fig. 8.2). Bahrain and UAE indicated that the CP approval time could be reduced by all sectors following the exact rules and regulations. In addition, if the verifications were carried out at the time when the applications were submitted, the timeline could be reduced. UAE also added that another way would be for the reviewing country to send the applications with preliminary assessment before the committee meets for their discussion as the committee sees the assessment for the first time at their meeting. To reduce the timeline further, Oman stated that the member states should not demand more requirements in order to avoid delaying the whole process; but they should encourage the sponsors to respond expeditiously to any such requests and by increasing the number of meetings. Kuwait stated that adopting mutual recognition from mature regulatory authorities would shorten the approval time. Saudi Arabia, on the other hand, advocated parallel national approvals to expedite the approval process. Bahrain, Oman, Saudi Arabia and UAE agreed that certain aspects of the registration procedure could be simplified to reduce the duration of the CP process. Bahrain stated that this could be achieved by developing a written policy for fast track registration, whilst Saudi Arabia and UAE advocated the implementation of electronic communication together with an online submission. Oman stated that the countries must consolidate all requests for additional information to be sent to the sponsors as one single batch instead of in several batches and at different times.
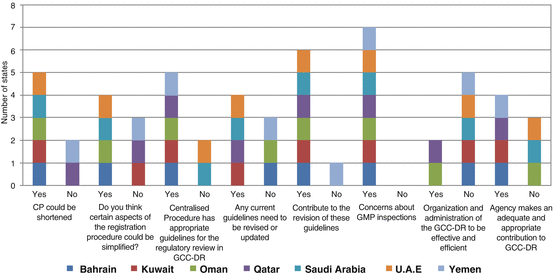

Fig. 8.2
Responses for the GCC authorities with regard to improving the GCC Centralised Procedure
Bahrain, Kuwait, Oman, Qatar, and Yemen agreed that the CP has appropriate guidelines for the regulatory review in GCC-DR, whilst UAE and Saudi Arabia report that currently the guidelines are not adequate. Bahrain, Kuwait, Qatar, Saudi Arabia, and UAE indicated that some of the existing guidelines need to be revised and/or updated. The areas recommended include GMP inspections, generics registration, contract manufacturing and biological/biosimilar registration. These guidelines should also be revised on a regular basis according to international requirements. All the member countries with the exception of Yemen indicated that they would be willing to contribute to the revisions of CP guidelines.
All member countries indicated their concerns about GMP inspections. Six of the member countries reported on the quality of expertise for GMP inspectors as well as the quality of the reports. UAE cited other causes, which included the inconsistency of the inspections and decision-making with regard to classification of defects and time given for corrective actions and their follow-up.
Only Qatar and Oman indicated that the organisation and administration of the GCC-DR are effective and efficient. Bahrain, Kuwait, Saudi Arabia, UAE, and Yemen reported that the organisation and administration of the GCC-DR is not effective or efficient. Bahrain, Saudi Arabia, UAE and Yemen cited the voting system as the contributing factor to the ineffectiveness, which in turn affects the efficiency of the GCC-DR system, whilst Kuwait cited that choosing the countries to conduct the evaluation affected the effectiveness and efficiency. In addition, Kuwait indicated that there is political pressure in some Gulf states for special consideration to be given to local manufacturers. Bahrain also added that the SOP for the voting system, which requires total agreement between all member states, should be written into the policy document.
Assessment of Centralised Procedure
In general the GCC Medicine Regulatory Authorities were satisfied with the GCC-DR assessment procedure, implementation of the regulatory guidelines and the availability of the scientific and technical expertise as is shown in Fig. 8.3. However, only two member countries (UAE and Yemen) stated that the quality of the CP decision-making is better than that of their national system, whilst four members (Bahrain, Kuwait, Oman and Qatar) indicated that it is about the same as their national system. On the other hand, Saudi Arabia indicated that the quality of the CP decision-making is worse than that of their national system, and this is certainly a cause of concern that needs to be further investigated to ascertain the root cause.
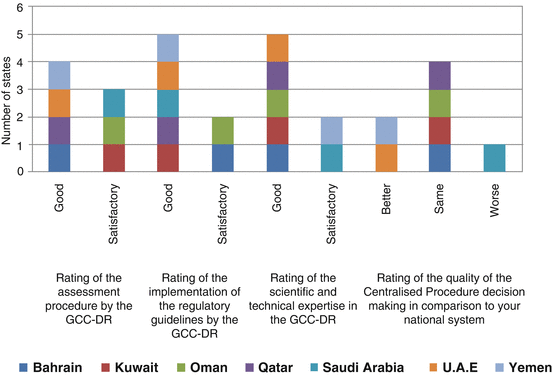

Fig. 8.3
Member states assessment of the Centralised Procedure
The GCC-DR Secretariat activities were also evaluated to ascertain the quality and deficiency, if any, within the organisation. Five member countries (Bahrain, Kuwait, Oman, Qatar and UAE) indicated that the level of interactions between the GCC-DR Secretariat and the GCC-DR Committee was good to excellent. Only two member countries (Saudi Arabia and Yemen) stated that it was only satisfactory. However, all member states, with the exception of Qatar, indicated that the GCC-DR Secretariat’s support to the GCC-DR Committee should be increased (Fig. 8.4).
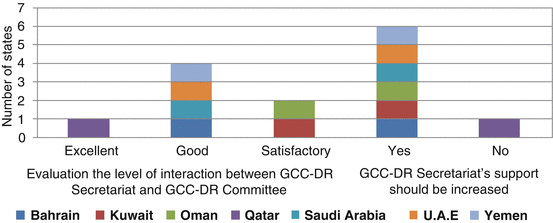

Fig. 8.4
Level of interaction between GCC-DR Secretariat and the Committee
The member states added that the Secretariat’s support should be enhanced through increasing the number of staff and improving the follow-up system across the member states.
They also recommended that there should be expert pharmacists at the Secretariat’s office to validate the submitted files, and this would ensure that the approval process is streamlined. This view was also reiterated by another member country, which stated that the Secretariat needed to include one or two pharmacists who have enough experience in drug registration and GMP inspections. Furthermore, since it is an administrative issue, more scientific staff is needed to organise the dossiers’ distribution, follow up the status of the companies, and communicate with other GCC members.
The key issues explored here centred on the allocation of resources to various activities carried out at the national or centralised level. All member states indicated that limited resources did pose moderate problems for their agency in relation to their activities with the Centralised Procedure. However, only Kuwait, Qatar, Saudi Arabia and UAE indicated that they did not lack the expertise required for the assessment of the Centralised Procedure applications. Bahrain, Oman and Yemen stated that they did lack the necessary expertise and that they would be able to overcome this issue by increasing their budget and engaging the resources required. Further information was gathered on how the GCC states evaluated their allocation of resources depending on the various CP activities. Only Oman, Saudi Arabia and UAE conducted GMP inspections at both the national and centralised levels (Fig. 8.5). Generally there was an increasing trend for the conduct of GMP inspections at a centralised level.
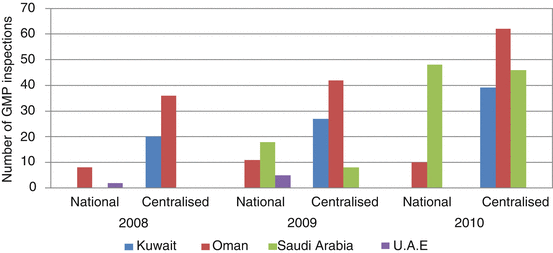

Fig. 8.5
Number of GMP inspections conducted by GCC (2008–2010)
UAE conducted a total of seven GMP inspections at a national level for 2008 and 2009 (Fig. 8.5). Oman conducted a total of 29 GMP inspections at a national level between 2008 and 2010, whereas Saudi Arabia conducted 66 such inspections for 2009 and 2010. It is obvious that there is an increasing trend in the conduct of GMP inspections at a national level over the 3 year period (2008–2010). More GMP inspections were carried out centrally in which the total inspections completed by Kuwait, Oman, Saudi Arabia and UAE for the 3 year period was 280. There is also an increasing trend towards central GMP inspections conducted by the four GCC countries from 56 in 2009 to 147 in 2010, which is an increase of more than 100 %.
Based on the data collected from Oman, Qatar and Saudi Arabia (Fig. 8.6), it could be ascertained that the number of product evaluation at a national level is more than those carried out under the CP. This indicates that most pharmaceutical companies prefer to receive approval at a national level rather than through the CP. This is further reinforced by the fact that half of the states indicated that the current CP evaluation system would not be sustainable due to the lack of resources, together with the current expertise and the heavy national workload.
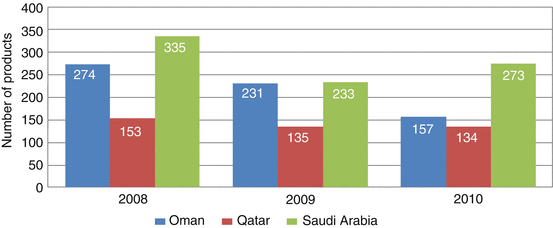

Fig. 8.6
Number of products evaluated by Oman, Qatar and Saudi Arabia (2008–2010)
There are a total of 250 internal and external reviewers available in the GCC. However, this number varied from state to state with Saudi Arabia having the largest number (121), whilst Yemen has the lowest number (11). With an increasing number of products to be evaluated, the number of reviewers within the GCC may not be sufficient to process the number of new products, thus resulting in the long delay experienced by the pharmaceutical companies.
Product evaluations in Oman, Saudi Arabia and Qatar at national and central registration reached a total of 1957 for the period 2008, 2009 and 2010 (Fig. 8.6). The highest number of product evaluations was carried out in the year 2008 with a total of 762 or about 40 % of the total number. Saudi Arabia has the highest total number of products evaluated (841), followed by Oman (662) and Qatar (422).
Furthermore, four member states did not provide the data for the product evaluated by them between 2008 and 2010 for the central registration. The data for Saudi Arabia was combined with that of the national data, as it was not differentiated when the data was first collected. There is no data available from Bahrain, Kuwait, UAE and Yemen for centralised product evaluation. In general, it could be ascertained that the number of products evaluated at a national level is more than those conducted within the Centralised Procedure.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


