Chapter 14 The Extracellular Matrix
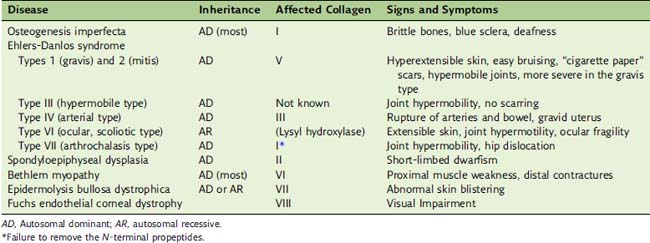
Connective tissues, in contrast, consist mainly of extracellular matrix. The mechanical properties of these tissues are determined by the composition of the extracellular matrix. Several building materials contribute to the extracellular matrix (Fig. 14.1):
Collagen is the most abundant protein in the human body
Collagen accounts for 25% to 30% of the total body protein in adults, making it the most abundant protein in the human body. As is evident from Table 14.1, collagen is most abundant in strong, tough connective tissues.
Table 14.1 Approximate Collagen Contents of Different Tissues, Expressed as Percentage of the Dry Weight
| Tissue | Collagen Content (%) |
|---|---|
| Demineralized bone* | 90 |
| Tendons | 80–90 |
| Skin† | 50–70 |
| Cartilage | 50–70 |
| Arteries | 10–25 |
| Lung | 10 |
| Liver | 4 |
* Bone from which the inorganic components (mostly calcium phosphates) have been removed by acid treatment.
† Mostly in the dermis. The major structural proteins of the epidermis are the keratins (see Chapter 13).
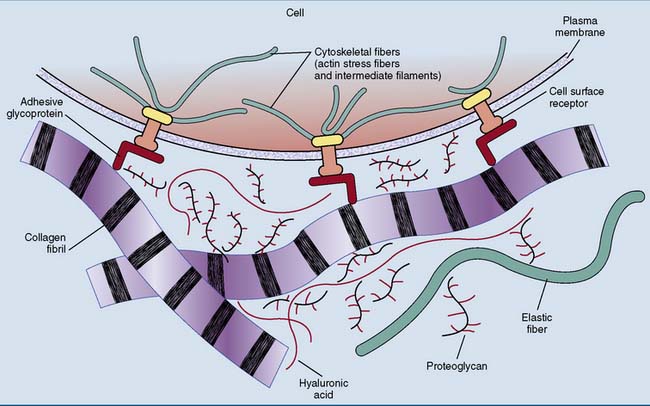
Humans have 28 different collagens and 42 genes encoding collagen chains. Some collagens form fibrils, but others, including the important type IV collagen in basement membranes, form extended networks. Others either are membrane proteins or are found on the surface of collagen fibrils (Table 14.2).
Tropocollagen molecule forms a long triple helix
The basic structural unit of collagen fibrils, the tropocollagen molecule, consists of three intertwined polypeptides (Fig. 14.2). In type I collagen, this three-stranded rope contains two different polypeptides, each with about 1050 amino acids: two copies of the α1(I) chain and one copy of the α2(I) chain. The structural formula is [α1(I)]2α2(I). These polypeptides have very unusual amino acid sequences, with glycine in every third position.
Each of the three polypeptides in tropocollagen forms a polyproline type II helix, which is very different from the familiar α-helix (see Chapter 2). The α-helix is a compact right-handed helix with 3.6 amino acids per turn and a rise per amino acid of 0.15 nm. The polyproline helix, however, is an extended left-handed helix with three amino acids per turn. With a rise of 0.30 nm per amino acid, it is twice as extended as the α-helix.
The three helical polypeptides of the tropocollagen molecule are wound around each other in a right-handed triple helix. Like the β-pleated sheet (see Chapter 2), this superhelical structure is held together by hydrogen bonds between the peptide bonds of the interacting polypeptides. The contacts are formed by that edge of the polyproline helix that has the glycine residues. Only glycine is small enough to permit close contact between the polypeptides. The whole molecule has a length of 300 nm and a diameter of 1.5 nm.
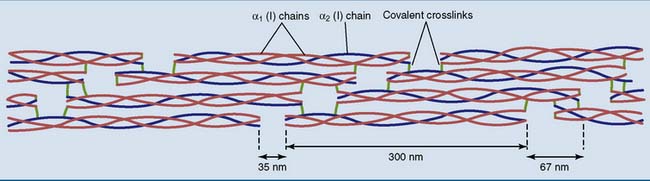
Collagen fibrils are staggered arrays of tropocollagen molecules
Collagen types I, II, III, V, and XI form cross-striated fibrils with diameters between 10 and 300 nm and a length of many hundreds of micrometers, containing hundreds or even thousands of tropocollagen molecules in cross-section. The tropocollagen molecules in the fibrils form a characteristic staggered array in which the end of one molecule extends 67 nm beyond that of its neighbor and with gaps of approximately 35 nm between the ends of successive molecules (Fig. 14.3). This staggered array gives collagen a characteristic cross-striated appearance under the electron microscope. More often than not, a single fibril contains more than one type of collagen.
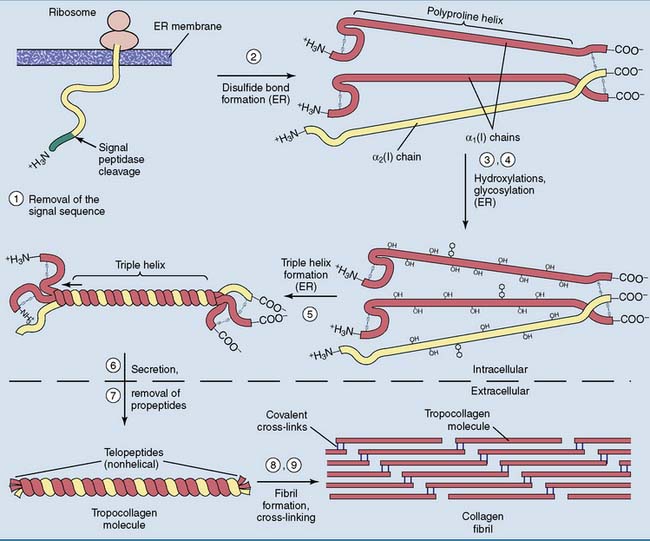
Collagen is subject to extensive posttranslational processing
Like all extracellular proteins, collagen is processed through the secretory pathway (see Chapter 8). The ribosomes on the rough endoplasmic reticulum (ER) synthesize pre-procollagen, which contains amino- (N-) and carboxyl- (C-) terminal propeptides in addition to the 1050 amino acids of tropocollagen. The propeptides have neither the unusual amino acid composition nor the triple-helical structure of tropocollagen. In the α1(I) chain of type I collagen, the propeptides measure approximately 170 amino acids at the amino end and 220 at the carboxyl end. The propeptides are needed to initiate the formation of the triple helix in the ER and to prevent premature fibril formation.
The steps in the processing of type I collagen (Fig. 14.4) are as follows:
Many genetic defects of collagen structure and biosynthesis are known
Mutations in the type I collagen genes cause bone diseases because virtually all of the collagen in bone is type I collagen (see Clinical Example 14.2). Most other tissues contain type I collagen along with type II (cartilage) or type III collagen (skin, blood vessels, hollow viscera).
Ehlers-Danlos syndrome typically presents with stretchy skin and loose joints. The “India rubber man” who could bend and twist himself in incredible shapes and package himself into tiny boxes had Ehlers-Danlos syndrome. The price for this virtuosity is a fragile skin that bruises easily. Even small wounds heal poorly, with the formation of characteristic “cigarette paper” scars. The classic forms are caused by defects in type V collagen, but numerous other clinical types are caused by different molecular lesions (Table 14.3).
Type VII collagen forms anchoring fibrils at the dermal-epidermal junction that anchor the basement membrane to the underlying dermis. The absence of this collagen causes the dystrophic variety of epidermolysis bullosa. Its clinical manifestations are similar to those of the keratin defects described in Chapter 13.
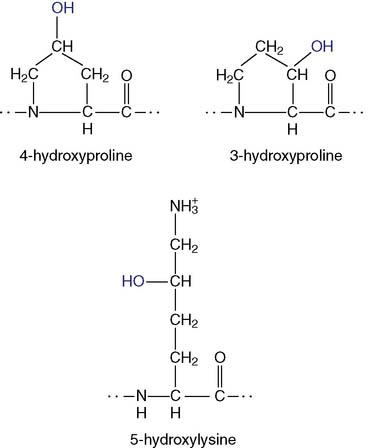

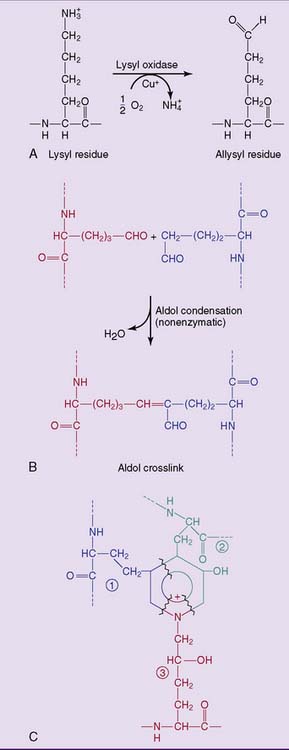
 , hydroxyallysine
, hydroxyallysine  , and hydroxylysine
, and hydroxylysine  .
.