The Esophagus
Danielle H. Carpenter
Elizabeth M. Brunt
I. NORMAL ANATOMY. The esophagus, a tubular structure that connects the pharynx to the stomach, is composed of cervical, thoracic, and abdominal segments. It begins at the level of the cricoid cartilage and ends at the gastroesophageal junction (GEJ). The GEJ is the junction of the tubular esophagus and the saccular stomach, and is distinct from the squamocolumnar junction.
By endoscopy, the esophagus is measured beginning 16 cm distal to the incisors and extends 35 to 40 cm to the GEJ. Precise anatomic location within the esophagus is a significant parameter in the differential diagnoses of various pathologic processes, as well as for staging squamous carcinoma.
The esophageal mucosa is composed of stratified squamous epithelium that extends distally to the squamocolumnar junction; this overlies paucicellular lamina propria and is delimited by thin muscularis mucosae that have a rich network of lymphatics (the latter allows for early metastases of relatively superficial malignancies). The deeper submucosa also has a rich lymphovascular network as well as submucosal glands connected to the lumen by ducts. The deep muscularis propria is composed of an inner circular layer and outer longitudinal layer; the proximal third of the muscularis is striated, the distal third is smooth muscle, and the middle third is a mixture. There is no serosal surface on the esophagus, but rather an adventitia.
II. GROSS EXAMINATION AND TISSUE HANDLING
A. Endoscopic biopsy. The standard endoscopic biopsy consists of several small (1 to 5 mm) unoriented pieces of mucosa with varying amounts of attached muscularis mucosae. In some cases, the endoscopist may use “jumbo forceps” to obtain larger fragments (4 to 8 mm); submucosa may be present in these biopsies. All fragments are submitted and three hematoxylin and eosin (H&E)-stained slides are examined.
B. Endoscopic mucosal resection (EMR). EMR is a more conservative approach than esophagectomy for resection of superficial malignant and premalignant lesions. These en bloc resections of 1 to 2 cm lesions are obtained by elevation of the mucosa with submucosal saline injection, followed by removal of the mucosa with variable amounts of attached submucosa. The specimens range from 1 to 4 cm in the longest dimension and up to 1 cm in thickness.
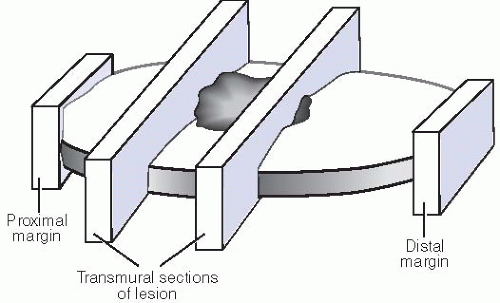
EMR specimens are carefully pinned flat for fixation, all deep and radial margins inked, and the specimen serially sectioned and submitted entirely to assess not only the mucosal lesion, but also the deep and radial margins (Fig. 12.1). Initially, at least three H&E levels are evaluated.
C. Esophagectomy. Esophagectomy specimens consist of esophagus, proximal stomach, and attached soft tissue with lymph nodes. The radial soft tissue margins are inked and the specimen opened longitudinally, avoiding transection of the lesion if possible; the specimen is pinned flat for fixation. The pertinent gross measurements include the overall length, diameter, and thickness of the esophagus and stomach; length, width, and thickness of the lesion and other mucosal abnormalities; and location of the lesion in relation to the proximal, distal, and radial margins, as well as in relation to the GEJ. The general appearance of the lesion (polypoid, ulcerated, and indurated) is important, as is that of the surrounding mucosa.
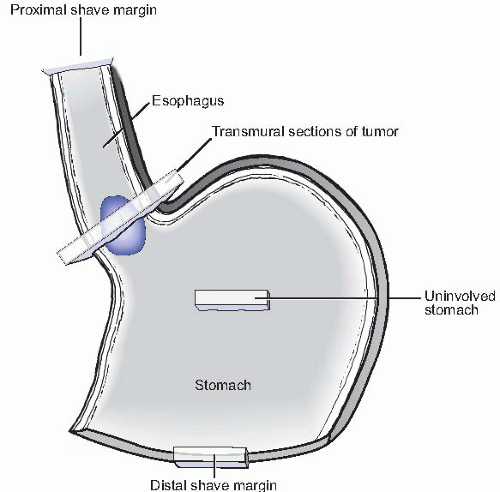
The standard sections (Fig. 12.2) should include a shave or en face section of the proximal esophageal margin (often submitted for frozen section and assessed intraoperatively) and distal stomach margin. A full thickness section of the lesion at its deepest point of invasion including the inked adventitial (radial) margin should also be taken; sections of tumor with closest adjacent proximal and distal margins should also be submitted as appropriate. When the tumor closely approximates the distal margin, the distal margin is entirely submitted radially to best assess distance from tumor to margin. In instances of preoperative radiation and/or chemotherapy, the lesion may be difficult to visualize grossly, and only a shallow ulcer or induration may be present; in such cases, the entire area should be serially sectioned and submitted for histologic examination. All lymph nodes visualized or palpated in the attached soft tissue are dissected and submitted.
III. NONNEOPLASTIC PROCESSES
A. Benign histologic changes
1. Glycogenic acanthosis. This asymptomatic, incidental finding is grossly characterized by white nodules or plaques and can be found throughout the length of the esophagus. Microscopically, glycogenic acanthosis is seen as a squamous epithelial layer thickened by clear epithelial cells in which the cytoplasm is distended by glycogen accumulation. The H&E appearance is characteristic, although the presence of glycogen can be confirmed with a Periodic acid-Schiff (PAS) stain.
2. Gastric heterotopias. Also known as the “inlet patch,” this lesion is a well-circumscribed, small (<2 cm) patch of gastric mucosa in the cervical
esophagus. Histologically, it can be antral, fundic, or cardiac type mucosa and is often inflamed (e-Fig. 12.1).*
3. Ectopic tissue. Other ectopic tissues have been described in the cervical esophagus including thyroid and parathyroid. Sebaceous glands have been described at all levels of the esophagus; they may be a type of metaplasia rather than ectopia.
B. Mucosal injury
1. Reflux esophagitis. In patients with gastroesophageal reflux disease (GERD), the distal esophagus may have a range of appearances from minimal erythema, to hemorrhage with ulceration. Histologically, the damage from the GERD is seen as intraepithelial inflammation with reactive changes (e-Figs. 12.2 to 12.4) characterized by an increased basal layer, elongation of the papillae, and intra- and intercellular edema; in severe cases, however, erosion can be observed as well.
Chronic gastroesophageal reflux with mucosal injury can also lead to the development of intestinal metaplasia (Barrett esophagus); this is discussed under section on “Epithelial Neoplasms and Preneoplasms.”
2. Chemical injury. Secondary to exposure to damaging or caustic agents, chemical injury is seen as mucosal damage ranging from mucosal erythema and
friability, to frank necrosis with perforation. Histologically, there is epithelial necrosis with or without inflammation, although usually to a lesser extent than would be expected for the degree of epithelial damage present.
TABLE 12.1 Clinicopathologic Features of Eosinophilic and Reflux Esophagitis
Eosinophilic esophagitis
Reflux esophagitis
Age
More common in children
More common in adults
Sex
More common in male
Equally in both sexes
Main symptom
Dysphagia
Heartburn
Endoscopic findings
Stricture, ring
Variable
Involvement
Entire esophagus
Distal esophagus
Microscopic finding
>20 eosinophils/hpf; eosinophilic microabscesses
<5 eosinophils/hpf
Therapy
Topical steroids
Anti-reflux
Complications
Stricture
Barrett esophagus; stricture
3. Pill esophagitis is usually a focal irregular erosion in areas of the esophagus where there is a natural external compression. Certain patients (elderly) with certain habits (taking pills without liquid) and certain medications (e.g., tetracycline, NSAIDs, large pills) are most commonly associated and the diagnosis can usually be made from a careful history. Histologically, the findings include inflammation, reactive changes, and erosion, and may include foreign pill material (e-Figs. 12.5 and 12.6).
4. Radiation injury. Acute radiation injury can share many features of other esophagitides and is usually clinically diagnosed due to its temporal relation to radiation therapy. Chronic radiation injury can be subtle clinically and show pale mucosa, a stricture, or chronic ulceration. Histologically, the findings include vascular changes consisting of ectasia and intimal fibrosis with resultant ischemic damage and erosion, as well as radiation fibroblasts and atypically enlarged (nucleus and cytoplasm) stromal cells (e-Figs. 12.7 to 12.9).
5. Eosinophilic esophagitis (EE). Clinically, EE shows a ridged or furrowed esophagus, and commonly involves the midesophagus. The histologic findings in EE may overlap with reflux esophagitis, although the intraepithelial inflammation is predominantly eosinophils (>20/hpf) and eosinophilic microabscesses are often seen (e-Figs. 12.10 to 12.12). Since reflux predominantly affects the distal esophagus, biopsies of both mid and distal esophagus are standard in suspected cases of EE. Table 12.1 presents differentiating clinicopathologic features of EE and reflux. The differential diagnosis of EE also includes hypereosinophilic syndrome and eosinophilic gastroenteritis, an allergic reaction to specific food antigens or drugs, or parasitic infection.
6. Graft versus host disease (GVHD) is uncommon in the esophagus, but is similar to GVHD in the skin. The squamous mucosa may desquamate, and submucosal fibrosis and stricture formation may develop. Histologically, basal vacuolation and apoptosis, with or without lymphocytic infiltration, are noted.
7. Infectious esophagitis
a. Candida. Candidiasis is classically seen as white exudative patches over the length of the esophagus with relatively normal appearing intervening mucosa. Histologically, there is a necroinflammatory background with yeast and pseudohyphae present in the superficial epithelial layers (e-Figs. 12.13 and 12.14). PAS and Grocott’s methenamine silver (GMS) stains highlight infiltrative fungal organisms (e-Fig. 12.15).
b. Herpes simplex virus (HSV). The classic lesion of HSV esophagitis is shallow ulceration, ranging from millimeters to centimeters in diameter. Viral cytopathic change, characterized by multinucleation, molding of nuclei, and margination of chromatin, are seen most frequently at the edges of the ulcer (e-Figs. 12.16 to 12.18). Immunohistochemistry (IHC) is confirmatory in this regard.
c. Cytomegalovirus (CMV). Focal, discrete ulceration in the distal esophagus occurs in CMV esophagitis. Granulation tissue from the ulcer base (as opposed to the edge in HSV) is optimal for identifying viral cytopathic change in endothelial and stromal cells. Large eosinophilic nuclear and/or cytoplasmic inclusions can be seen on routine H&E stained sections, although immunostains for CMV can highlight or confirm the virus.
IV. BENIGN POLYPS
A. Fibrovascular polyps are elongate, often large (“giant fibrovascular polyp”), pedunculated intraluminal growths that can fill the esophagus and present as an intraoral mass if regurgitated. Histologically, these polyps are composed of an edematous, loose stroma with a rich vascular network covered by benign squamous mucosa.
B. Inflammatory polyps. Often related to GERD, inflammatory polyps are present near the squamocolumnar junction and may endoscopically resemble adenocarcinoma. However, histologically they are benign and composed of inflamed squamous and/or foveolar mucosa.
C. Squamous papilloma. Also often related to GERD, squamous papillomas can have a filiform appearance microscopically, if not grossly. Delicate fibrovascular cores are covered by benign reactive squamous epithelium with varying degrees of inflammation (e-Fig. 12.19). Although rare cases of virus-related papillomas of the esophagus have been reported in immunocompromised patients, squamous papillomas are most often seen in patients with GERD.
V. EPITHELIAL NEOPLASMS AND PRENEOPLASMS. The current World Health Organization (WHO) histologic classification of esophageal tumors is given in Table 12.2. The AJCC Staging guidelines for esophagus and esophagogastric junction are applied to primary esophageal and esophagogastric junction mucosal tumors, including the proximal 5 cm of stomach (Table 12.3).
A. Squamous dysplasia. Endoscopic evidence of squamous dysplasia may be subtle and most likely identified in association with plaque-like, mass-forming lesions. Dysplastic squamous mucosa has cells with enlarged, hyperchromatic nuclei and increased nuclear to cytoplasmic ratios, as well as overall dysmaturity of the epithelium. Unlike squamous carcinoma, dysplasia is limited by the basement membrane; however, unlike benign reactive changes, dysplasia lacks cytologic uniformity and orderly epithelial maturation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree