Chapter 10 THE ENDOCRINE PANCREAS
Introduction
The endocrine pancreas consists of the islets of Langerhans, which are scattered between the acini and ducts of the exocrine part of the gland. The clinical syndromes related to the pathology of the islets of Langerhans result from either underproduction or overproduction of hormones normally produced by the cells forming the islets. The most important disease resulting from the underproduction of insular hormones is diabetes mellitus (DM). The syndromes related to overproduction of insular hormones are typically caused by tumors, which are also discussed here (Fig. 10-1). These tumors are relatively rare, accounting for 1% to 3% of all pancreatic neoplasms.
Normal Structure and Function
ANATOMY AND HISTOLOGY
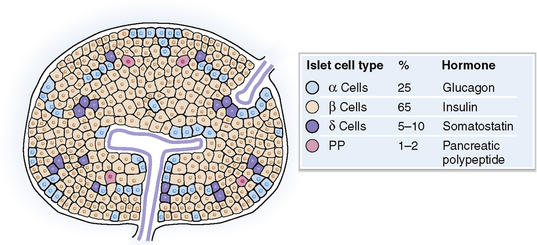
The islets of Langerhans consist of several cell types (Fig. 10-2):
 Alpha (α) cells, which secrete glucagon
Alpha (α) cells, which secrete glucagon
 Beta (β) cells, which secrete insulin, proinsulin, C peptide, and amylin
Beta (β) cells, which secrete insulin, proinsulin, C peptide, and amylin
 Delta (δ) cells, which secrete somatostatin
Delta (δ) cells, which secrete somatostatin
 F cells (PP cells), which secrete pancreatic polypeptide but only in some islets
F cells (PP cells), which secrete pancreatic polypeptide but only in some islets
PHYSIOLOGY
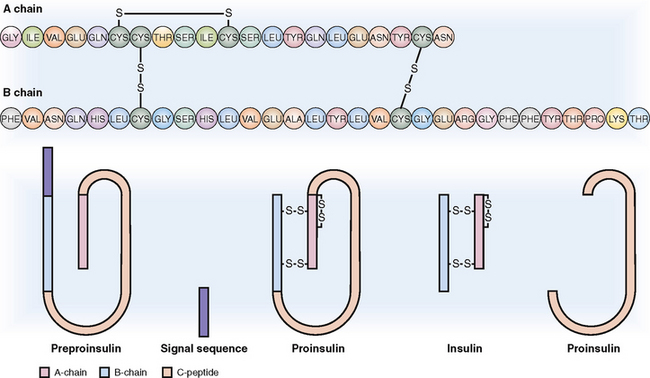
Insulin is a polypeptide formed from inactive precursors.
Insulin is a short polypeptide composed of 51 amino acids arranged into two chains linked together by two disulfide bonds (Fig. 10-3). Insulin synthesis occurs in several steps:
 Preproinsulin. The original gene product, called preproinsulin, is a polypeptide that contains the A and B chains of insulin linked together with a long C polypeptide and a signal sequence on the N-terminal.
Preproinsulin. The original gene product, called preproinsulin, is a polypeptide that contains the A and B chains of insulin linked together with a long C polypeptide and a signal sequence on the N-terminal.
 Proinsulin. It is formed in the cisterns of the rough endoplasmic reticulum through the cleavage of the signal sequence and the formation of the interchain and intrachain disulfide.
Proinsulin. It is formed in the cisterns of the rough endoplasmic reticulum through the cleavage of the signal sequence and the formation of the interchain and intrachain disulfide.
 Insulin. It is formed from proinsulin through the cleavage of the C peptide in the Golgi apparatus, from which it is transferred to the storage granules in the cytoplasm. From these granules insulin is released into the circulation by endocytosis. The circulating insulin has a short half-life (6 minutes), during which period it has the opportunity to bind to insulin receptors, predominantly in the liver, muscle, and fat cells. It is degraded by hepatic insulinase.
Insulin. It is formed from proinsulin through the cleavage of the C peptide in the Golgi apparatus, from which it is transferred to the storage granules in the cytoplasm. From these granules insulin is released into the circulation by endocytosis. The circulating insulin has a short half-life (6 minutes), during which period it has the opportunity to bind to insulin receptors, predominantly in the liver, muscle, and fat cells. It is degraded by hepatic insulinase.
Anatomy and Physiology
Glucagon Polypeptide hormone produced by insular alpha cells that regulates the metabolism of carbohydrates. It promotes glycogenolysis in the liver, thus causing hyperglycemia. It may be produced by some pancreatic endocrine tumors (glucagonomas).
Glucose Key component of carbohydrate metabolism, present in free form in the blood or complexed into oligo- and polysaccharides in tissues. The normal concentration of glucose in serum or plasma is 70 to 110 mg/dL (3.9–6.1 mmol/L).
Insulin Polypeptide hormone produced by insular beta cells that is involved in regulating the metabolism of carbohydrates, lipids, and proteins. It has many functions, the most important of which is the promotion of glycogenesis in the liver and the uptake and utilization of glucose in muscle, fat cells, and several other tissues, thus lowering the blood concentration of glucose. It may be produced by some pancreatic endocrine tumors (insulinomas).
Insulin receptor Tyrosine kinase-linked cell membrane receptor that binds circulating insulin. Its activation leads to metabolic changes, the most important of which is increased influx and utilization of glucose. Defective function of insulin receptors leads to diabetes mellitus type 2.
Islets of Langerhans Endocrine part of the pancreas. Each islet is composed of several cell types: alpha cells that secrete glucagon, beta cells that secrete insulin, delta cells that secrete somatostatin, and F cells that secrete pancreatic polypeptide.
Pancreatic polypeptide Polypeptide secreted by endocrine and exocrine pancreatic cells and intestinal cells. It inhibits the secretion of pancreatic enzymes and the contraction of the gallbladder.
Somatostatin Polypeptide hormone produced by insular delta cells and intestinal endocrine cells. It inhibits the release of several other hormones, such as growth hormone, glucagons, insulin, and gastrin. It may be produced by some pancreatic or intestinal endocrine tumors (somatostatinomas).
Vasoactive intestinal polypeptide Polypeptide hormone widely distributed in the body, but most prominently found in the central nervous system and intestines. It leads to intestinal vasodilatation and hypermotility, as well as gastrointestinal water and electrolyte secretion. It may be produced by some pancreatic endocrine tumors (VIPomas).
Diseases of Endocrine Pancreas
Diabetes mellitus Metabolic syndrome characterized by hyperglycemia related to absolute or relative insulin deficiency or tissue resistance to insulin.
Gastrinoma syndrome Metabolic syndrome also known as Zollinger-Ellison syndrome, caused by gastrin-secreting tumors, most often located in the head of the pancreas. It is characterized by hypergastrinemia, gastric acid hypersecretion, and peptic ulcers that are often multiple and found in atypical locations. It is also sometimes characterized by a resistance to conventional antiulcer therapy.
Glucagonoma syndrome Metabolic syndrome caused by glucagonoma (i.e., a glucagon-secreting islet cell tumor composed of alpha cells). The syndrome includes mild diabetes, migratory erythematous skin necrosis, anemia, thrombosis, and a predisposition to bacterial infections.
Hyperglycemia Increased concentration of glucose in serum (>120 mg/dL), typically seen in diabetes mellitus as well as in a variety of endocrine diseases (e.g., Cushing’s syndrome, hyperthyroidism) and acute pancreatitis. Any type of shock increases serum glucose levels. It may be also drug-induced (e.g., by thiazide diuretics, phenytoin, and epinephrine).
Hypoglycemia Decreased concentration of glucose in serum (<40 mg/dL). Reactive hypoglycemia may occur after feeding (postprandial hypoglycemia). Fasting hypoglycemia is typically found in association with insulinoma, insulin abuse, or insulin overdose (in diabetic patients), but may be also found in association with some glycogen-storing or -consuming tumors and in severe systemic diseases.
Insulinoma syndrome Syndrome caused by insulinoma (i.e., an insulin-secreting islet cell tumor composed of beta cells). It is characterized by hypoglycemia, sweating, nervousness, lethargy, or fainting that may occasionally progress to hypoglycemic coma.
Islet cell tumors Group of endocrine tumors originating from the islets of Langerhans. These tumors may be benign or malignant, solitary or multiple, hormonally active or inactive. On the basis of the type of cells that form these tumors they are classified as insulinomas (most common), glucagonomas, gastrinomas, somatostatinomas, vasoactive intestinal polypeptide-secreting tumors (VIPomas), or pancreatic polypeptide-secreting tumors (PPomas). Histologically they resemble carcinoid tumors of the gastrointestinal and respiratory tract. By electron microscopy they contain dense membrane-bounded granules. Highly malignant tumors may resemble small-cell (oat cell) carcinomas of the lung.
Somatostatinoma syndrome Metabolic syndrome caused by somatostatinomas (i.e., somatostatin-secreting islet cell tumors). It is characterized by mild diabetes, steatorrhea, gastric hypochlorhydria, and gallstones.
VIPoma Metabolic syndrome, also known as Verner-Morrison syndrome, caused by VIPomas (i.e., pancreatic islet tumors composed of vasoactive polypeptide [VIP]-secreting cells). It is characterized by watery diarrhea, hypokalemia, and gastric hypochorhydria or even achlorhydria.
Secretion of insulin and glucagon is regulated by blood glucose, other metabolites, and some hormones.
Factors that regulate the secretion of insulin can be classified as stimulatory and inhibitory.
 Stimulation of insulin release. High blood glucose directly stimulates beta cells, and it is thus the most important stimulus for insulin production and release. Other food constituents and metabolites, such as amino acids and fatty acids, may stimulate insulin release directly, but most often their effect is indirect, since these metabolites affect the utilization or release of glucose into the bloodstream. Intestinal secretin, released on feeding, stimulates insulin release. Cortisol and growth hormone induce peripheral insulin resistance and increase blood glucose concentration, thus stimulating the release of insulin.
Stimulation of insulin release. High blood glucose directly stimulates beta cells, and it is thus the most important stimulus for insulin production and release. Other food constituents and metabolites, such as amino acids and fatty acids, may stimulate insulin release directly, but most often their effect is indirect, since these metabolites affect the utilization or release of glucose into the bloodstream. Intestinal secretin, released on feeding, stimulates insulin release. Cortisol and growth hormone induce peripheral insulin resistance and increase blood glucose concentration, thus stimulating the release of insulin.
 Inhibition of insulin release. Insulin secretion is reduced physiologically during starvation and decreased food intake. Acute inhibition of insulin secretion during stress or trauma is mediated by epinephrine, which stimulates release of glucose from the liver and fatty acids from fat tissues, but also acts on pancreatic beta cells to decrease their sensitivity to glucose. Somatostatin has a paracrine inhibitory effect on insulin secretion.
Inhibition of insulin release. Insulin secretion is reduced physiologically during starvation and decreased food intake. Acute inhibition of insulin secretion during stress or trauma is mediated by epinephrine, which stimulates release of glucose from the liver and fatty acids from fat tissues, but also acts on pancreatic beta cells to decrease their sensitivity to glucose. Somatostatin has a paracrine inhibitory effect on insulin secretion.
 Stimulation of glucagon release. The most potent stimulus for glucagon release is low blood glucose concentration, as occurs in hunger. Epinephrine release during stress or trauma also promotes glucagon release. It also overrides the effects of excess glucose release from the liver, which acts directly on the insular cells. Amino acids also stimulate glucagon release, thus counteracting their stimulatory effect on insulin release.
Stimulation of glucagon release. The most potent stimulus for glucagon release is low blood glucose concentration, as occurs in hunger. Epinephrine release during stress or trauma also promotes glucagon release. It also overrides the effects of excess glucose release from the liver, which acts directly on the insular cells. Amino acids also stimulate glucagon release, thus counteracting their stimulatory effect on insulin release.
 Inhibition of glucagon release. The most important inhibitor is high blood glucose concentration. Ketone bodies and free fatty acids have the same effect.
Inhibition of glucagon release. The most important inhibitor is high blood glucose concentration. Ketone bodies and free fatty acids have the same effect.
Insulin and glucagon regulate the intermediary metabolism of carbohydrates, lipids, and proteins.
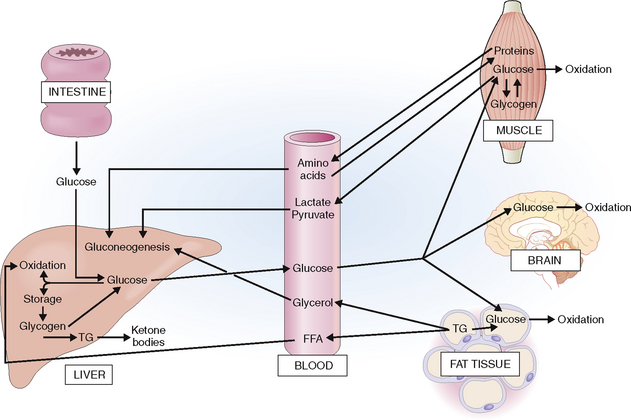
The actions of insulin and glucagons are closely interlinked. Insulin has predominantly anabolic effects and is “anticatabolic,” whereas glucagon has predominantly catabolic effects (Fig. 10-4). This is reflected in the blood insulin-to-glucagon ratio, which is high in the fed condition and low in a fasting state.
Insulin acts predominantly on liver, muscle, and fat tissue.
 Liver. In the liver it promotes storage of glucose in the form of glycogen by promoting glycogen formation and its lysis into glucose. The net outflow of glucose from the liver is reduced. Excess glucose is used for the synthesis of lipids, which are stored in liver cells in the form of triglycerides (TGs). Insulin also affects lipid metabolism by inhibiting ketogenesis and promoting synthesis of very low density lipoproteins (VLDLs). When VLDLs are released into the bloodstream they are taken up by muscle cells or fat cells and stored or used for energy production. Insulin stimulates the uptake of amino acids into liver cells.
Liver. In the liver it promotes storage of glucose in the form of glycogen by promoting glycogen formation and its lysis into glucose. The net outflow of glucose from the liver is reduced. Excess glucose is used for the synthesis of lipids, which are stored in liver cells in the form of triglycerides (TGs). Insulin also affects lipid metabolism by inhibiting ketogenesis and promoting synthesis of very low density lipoproteins (VLDLs). When VLDLs are released into the bloodstream they are taken up by muscle cells or fat cells and stored or used for energy production. Insulin stimulates the uptake of amino acids into liver cells.
 Muscle. In the muscles insulin promotes the uptake of glucose from the blood and glycogen synthesis. It also inhibits glycogen phosphorylase and slows down glycogenolysis. Insulin stimulates the entry of amino acids into muscle cells and protein synthesis.
Muscle. In the muscles insulin promotes the uptake of glucose from the blood and glycogen synthesis. It also inhibits glycogen phosphorylase and slows down glycogenolysis. Insulin stimulates the entry of amino acids into muscle cells and protein synthesis.
 Fat tissue. Insulin stimulates glucose uptake and promotes TG storage by promoting the uptake of fat and fatty acid esterification and by inhibiting lipolysis of TGs.
Fat tissue. Insulin stimulates glucose uptake and promotes TG storage by promoting the uptake of fat and fatty acid esterification and by inhibiting lipolysis of TGs.
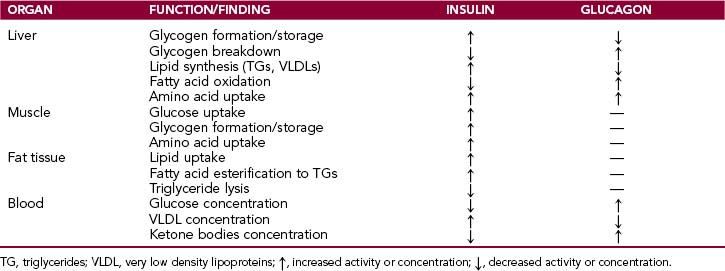
The main effects of insulin and glucagon are listed in Table 10-1.
Clinical and Laboratory Evaluation of Diseases of the Endocrine Pancreas
FAMILY AND PERSONAL HISTORY
Except for diabetes mellitus (DM), which affects millions of people worldwide, other diseases of the endocrine pancreas are relative rare. Hence, in this chapter the major emphasis is on DM and its complications. The risk factors for DM and other less common pancreatic endocrine disorders can be discovered by careful taking of the patient’s history and are listed in Table 10-2.
Table 10-2 Risk Factors for Diabetes Mellitus (DM) and Other Less Common Pancreatic Endocrine Disorders
| TYPE OF RISK FACTOR | SPECIFIC DISEASES–RISK FACTOR ASSOCIATIONS |
| Hereditary factors | |
| Social/nutritional factors | Obesity-related type 2 DM |
| Other diseases of the pancreas | Acute and chronic pancreatitis and secondary DM |
| Other endocrine diseases | Hyperglycemia due to Cushing’s syndrome, or acromegaly |
| Medical and surgical procedures | DM after resection of the pancreas for tumors |
| Drugs | Drug-induced hyperglycemia or DM |
| External mechanical factors | Pancreatic necrosis due to seat belt trauma and secondary DM |
Increased incidence of type 2 diabetes in children and adolescents has prompted the ADA to recommend screening of children at age 10 years if the child has the following risk factors:
PHYSICAL EXAMINATION AND HISTORY OF PRESENT DISEASE
The most important clinical findings pointing to DM or other endocrine pancreatic disorders are:
Polyuria and polydipsia are common manifesting signs of diabetes mellitus.
It is important to note that polyuria may be related to excessive intake of water, as in people who have an urge to drink (“psychogenic polydipsia”), and other endocrine disorders (e.g., hyperparathyroidism and other forms of hypercalcemia). Many intracranial lesions interfering with the secretion of the antidiuretic hormone (ADH) may cause “cranial” diabetes insipidus. Several renal diseases and drugs and toxins that affect the kidneys may manifest as “nephrogenic diabetes insipidus” (Table 10-3).
| CLINICAL CONDITION | MECHANISM | FINDINGS |
| Psychogenic polydipsia | Water intake ↑ | Plasma sodium → or ↓ |
| Diabetes mellitus | Osmotic water loss | Plasma osmolality ↓ |
| Hyperparathyroidism | Osmotic water loss | Plasma/urine glucose ↑ |
| Cranial diabetes insipidus (reduced renal water reabsorption) | Lack of ADH | Plasma/urine calcium ↑ |
| Renal diseases | Inability to concentrate urine | Urine osmolality ↓ (<600 mOsm/kg) |
| Drugs (e.g., lithium) | Renal effects | Plasma osmolality ↑ (>300 mOsm/kg) |
| Heavy-metal toxicity | Renal effects | Other signs of kidney disease |
ADH, antidiuretic hormone; →, normal; ↓, reduced; ↑, increased.
Polyuria is a complication of hyperglycemia, which leads to glucosuria. Glucose in the urine “draws out” the water and thus leads to an osmotic polyuria. It is associated with a loss of fluid, minerals, and glucose in the urine. Dehydration may cause clinical symptoms such as dry skin and mucosal surfaces and reduced skin turgor. A hyperglycemia-related hyperosmolar state may cause blurry vision because of exposure of the lens and retina to hyperosmolar plasma. Reduced plasma volume (hypovolemia) may cause hypotension, syncope, and dizziness.
Polyphagia results from increased metabolic demands.
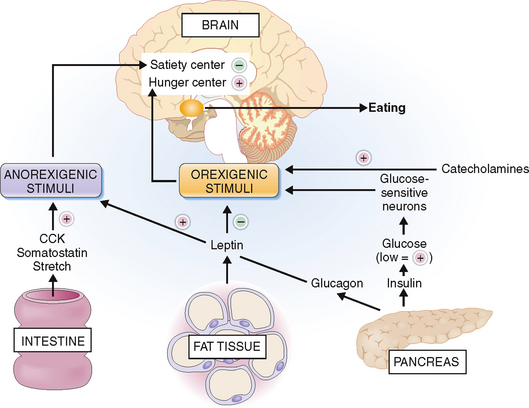
The reasons for polyphagia are not quite obvious but are directly or indirectly related to disturbances of the feeling of hunger. The eating behavior of each person is a function of hypothalamic centers known as the satiety and hunger centers (Fig. 10-5). These centers receive numerous impulses, which can be classified as anorexigenic (i.e., suppressing appetite) or orexigenic (promoting appetite). The secretion of orexins that act on the hunger center is stimulated by low glucose concentration in the blood. This correlates with the well-known fact that hypoglycemia, induced by exogenous insulin or insulin-producing tumors, manifests with hunger. Patients who have type 1 DM apparently lose the function of “glucose-sensitive neurons” and persistently secrete orexins. Another stimulus for overeating in type 1 DM might be a lack of glucagon or cholecystokinin (CCK), a pancreaticointestinal polypeptide that under normal circumstances inhibits hunger. The effects of CCK are seen only in the presence of an intact vagus nerve, which may be affected by diabetic neuropathy or the hyperosmotic state caused by hyperglycemia. Catecholamines, and especially norepinephrine, which are elevated in type 1 DM due to stress and poor glycemic control, also act by stimulating eating.