Chapter 13 The Cytoskeleton
The erythrocyte membrane is reinforced by a spectrin network
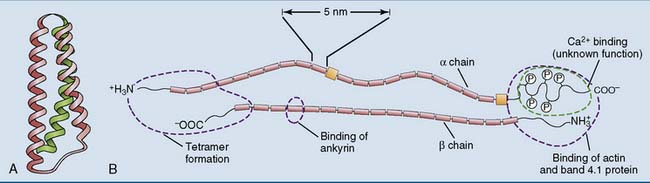
Erythrocytes travel about 300 miles during their 120-day life, part of this through tortuous capillaries in which they suffer mechanical deformation. They can survive this only because their membrane is reinforced by a meshwork of fibers formed by the proteins α-spectrin and β-spectrin. Each spectrin monomer consists of spectrin repeats, a domain of 106 amino acids that forms a coiled coil of three intertwined α-helices. It is repeated (with variations) 20 times in the α-chain and 17 times in the β-chain (Fig. 13.1).
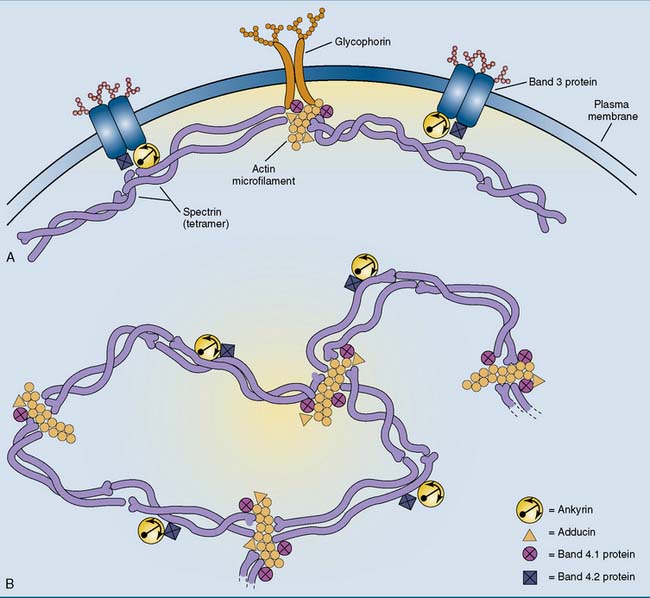
Spectrin forms an antiparallel dimer, with an α-chain and a β-chain lying side by side. These α-β dimers condense head to head to form a tetramer, which is a long, wriggly, wormlike molecule with a contour length of 200 nm and a diameter of 5 nm. The ends of the spectrin tetramer are bound noncovalently to short (35-nm) actin filaments. This interaction is facilitated by two other proteins: band 4.1 protein (so named after its migration in gel electrophoresis) and adducin. By binding several spectrin tetramers, the actin filaments form the nodes of a two-dimensional network that can be likened to a fishing net or a piece of very thin, flexible chicken wire (Fig. 13.2, B).
The spectrin network is anchored to the membrane by the peripheral membrane protein ankyrin, which itself is bound to the integral membrane protein band 3 protein. This binding is stabilized by band 4.2 protein (pallidin). The actin microfilaments are attached to the membrane mainly through band 4.1 protein and the integral membrane protein glycophorin. The erythrocyte membrane skeleton is important because inherited defects in its components give rise to hemolytic anemias (Clinical Example 13.1).
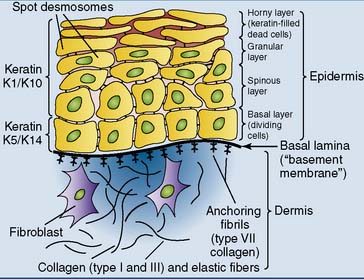
Keratins are the most important structural proteins of epithelial tissues
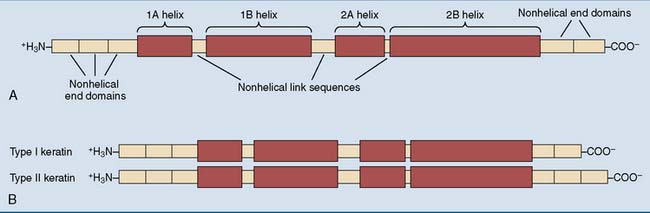
All keratins contain long stretches of α-helix interrupted by short nonhelical segments (Fig. 13.3, A). The two different types are the acidic (type I) and the basic (type II) keratins. Each comes in about 15 different variants. They form heterodimers, with a type I polypeptide forming a coiled coil with a type II polypeptide (Fig. 13.3, B). The α-helices of the two keratins make contact through hydrophobic amino acid side chains on one edge of each helix. Typical keratin fibrils contain between 12 and 24 of these heterodimers in a staggered array.
Different keratins are expressed in different cell types. The basal layer of the epidermis forms K14 as the major type I keratin and K5 as the major type II keratin. In the more mature cells of the spinous and granular layers, keratins K10 and K1 are the major type I and type II keratins, respectively (Fig. 13.4). Single-layered epithelia express keratins 18, 19, and/or 20 (type I) and keratins 7 and 8 (type II). Various other keratin pairs are expressed in the cells that form hair and nails.
Several intermediate filament proteins other than the keratins are expressed in various cell types (Table 13.1). All of them are dynamic structures that are assembled and disassembled continuously.
Table 13.1 Major Types of Intermediate Filament Proteins*
| Protein | Tissue or Cell Type |
|---|---|
| Keratin | Epithelial cells, hair, nails |
| Vimentin | Embryonic tissues, mesenchymal cells, most cultured cells |
| Desmin | Myocardium, at Z disk in skeletal muscle |
| Glial fibrillary acidic protein | Astrocytes, Schwann cells |
| Peripherin | Neurons of PNS |
| α-Internexin | Neurons of CNS |
| Neurofilament proteins (NF-L, NF-M, NF-H) | Neurons of CNS and PNS |
| Lamin | Nucleus of all nucleated cells. |
CNS, Central nervous system; PNS, peripheral nervous system.
* All of these proteins have the general structure depicted in Figure 13.3, for keratin.
The lamins are the only intermediate filament proteins that are found in the nucleus rather than the cytoplasm. They form a supporting fiber network under the nuclear envelope. During mitosis, the lamins become phosphorylated by the cell cycle–induced protein kinase Cdk1. This leads to the disassembly of the fibers and the collapse of the nuclear envelope (see Chapter 18).
Actin filaments are formed from globular subunits
The loose subunits are called G-actin (G for globular). They have a molecular weight (MW) of 42,000 and a nucleotide binding site that is occupied by ATP or ADP. These subunits can polymerize into a filament in which two strands are coiled gently around one another (Fig. 13.5). Microfilaments are dynamic structures that can be assembled and disassembled continuously.
Cells have a bloated bureaucracy of proteins to regulate the formation, growth, and dissolution of microfilaments. Some initiate the formation of a new microfilament, some anchor the filaments to membranes or cytoskeletal structures, and others bundle them into networks or parallel arrays (Table 13.2).
Table 13.2 Proteins That Regulate Actin Microfilaments
| Protein | Function |
|---|---|
| Thymosin | Binds free actin monomers, making them unavailable for polymerization |
| Profilin | Delivers actin monomers to growing microfilaments |
| ARP complex | Nucleates microfilaments at the − end |
| Formin | Binds to the + end of microfilaments, promotes elongation |
| Tropomyosin | Strengthens microfilaments, regulates their length |
 | Prevents myosin from binding to actin/tropomyosin |
 | Link microfilaments into a gel |
 | Link microfilaments into parallel bundles |
 | Link microfilaments to the plasma membrane |
| Cap Z | Caps and stabilizes the + end of microfilaments |
| Tropomodulin | Caps and stabilizes the − end of microfilaments |
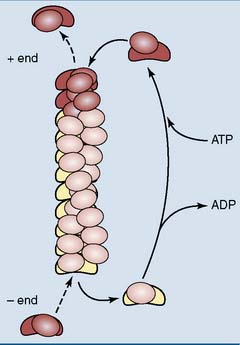
 , Actin monomer with bound ADP;
, Actin monomer with bound ADP;  , actin monomer with bound ATP.
, actin monomer with bound ATP.


