, Mohammed Al-Rubaie2, Stuart Walker3 and Sam Salek4
(1)
Regulatory Affairs Consultant Executive Directive, Kuwait Advancement for Conference and Exhibition Management, Kuwait, Kuwait
(2)
Ministry of Health, Directorate General of Pharmaceutical Affairs and Drug Control, Muscat, Oman
(3)
Founder of Centre for Innovation in Regulatory Science, London, UK
(4)
Department of Pharmacy School Life & Medical Sciences, University of Hertfordshire, Hatfield, UK
Introduction
Although an awareness of the important role of drug safety monitoring (pharmacovigilance) in the Gulf states has dramatically improved in recent years, communication of medicines benefits and harms between the various stakeholders, such as regulatory authorities, pharmaceutical industry, healthcare professionals and consumers, lags far behind. Dusetzina et al. (2012) described several methods that have been used by the United States Food and Drug Administration (US FDA) during the past two decades to communicate hundreds of drug risks discovered after market approval. Sharing information regarding adverse effects of medicinal products and communicating risks amongst stakeholders should be given high priority. Bahri and Harrison-Woolrych (2012) stated that the core of successful risk management lies in effective risk communication, and this applies to PV activities, risk management plans (RMPs) and risk minimisation activities. The flow of benefit-risk communication is from the regulatory authorities and pharmaceutical companies to other stakeholders such as healthcare professionals and patients/public and from healthcare professionals to patients. However, effective risk communication (RC) requires proactive approaches to protect the patients’ rights and to increase transparency in sharing information amongst the relevant stakeholders. The involvement of patients has also been considered the fundamental basis for identifying and communicating risks. Therefore, advanced authorities such as US FDA and European Medicines Agency (EMA) have established a framework for direct patient reporting of adverse drug reactions. The US FDA began in 2003 and EMA recommended it to its EU member states, and it has already started in the European (EU) region (McAuslane and Walker 2013).
Regulatory authorities around the world recognise the importance of communicating the outcome of the regulatory review effectively to accomplish the ultimate goal of promoting and protecting public health. Effective communication fosters the rational prescribing and use of medicines and increases the public awareness about the best method of safe consumption. It is the responsibility of the regulatory authorities to adopt effective pharmacovigilance (PV) and risk management strategies by educating regulators, healthcare professionals and individuals as to the importance of communicating risks to the health authorities. They are also responsible for establishing regulations to enhance drug safety monitoring and enforce legislations to support effective reporting of adverse events (AEs). Bouder (2011) indicated that effective risk communication becomes particularly challenging when regulatory authorities interact with various stakeholders simultaneously. In response to the changing societal information, Seligman and Osborne (2009) have signified the FDA’s evolving role in providing knowledge-based approaches aimed solely at the healthcare providers to communicate information directly to patients and the general public.
The responsibility of the pharmaceutical industry (PI) for communicating benefits and risks to the regulatory authorities, healthcare professionals and individuals cannot be overlooked (EMA 2013). They are the cornerstone of such information and must possess optimal communication skills to deliver the message to the recipient (healthcare professional, patients, regulators) in a clear, transparent evidence-based manner (Urushihara et al. 2014). In both the European Commission (EC) and the United States (US), legal requirements and public attitudes require the companies to disclose risk information to the targeted stakeholders. The results of a study on 399 patients presented by Cullen et al. (2006) stated that overall patients underrated the risk of their medications, which indicates a lack of knowledge and awareness about the effects of their medications. Another study by Corry et al. (2000), which examined hospital letters using a predetermined protocol, indicated that general practitioners and hospital doctors are not well informed about medications that should be monitored. This indicates a lack of communication between stakeholders, which is considered the backbone of PV systems and risk management plans (RMPs). Mammi et al. (2013) states that ‘pharmaceutical companies should be responsible for continuously monitoring the safety of their medicinal products for human use, for informing the authorities of any changes that might have an impact on the marketing authorization and for ensuring that the product information is kept up-to-date’.
Harmonisation of the Pharmacovigilance Practices
The time has arrived for the drug regulatory authorities in the six Gulf states to implement new harmonisation initiatives to strengthen their regulatory control of the quality, safety and efficacy of medicines before and after granting their marketing authorisation. Each country has established a national regulatory system and has been making an effort to keep up with the advancing regulatory developments around the world. Therefore, the six states have adopted harmonisation procedures to unify the regulatory processes by developing a set of guidelines and policies to follow (Alessa et al. 2011). The GCC Central Registration Committee is one of the regional regulatory harmonisation attempts, which was implemented in 1999 to unify the pre-marketing assessment and marketing approval process in the Gulf region. The new Arab Common Pharmacovigilance Guidelines is the latest harmonisation initiative that has been adopted in the Arab world (including the GCC region). The guidelines were developed by Arab states that already have existing national pharmacovigilance strategies, such as Saudi Arabia, Egypt and Jordan, and were based on the European pharmacovigilance guidelines with fine-tuning of some areas to suit the cultural and socioeconomic status of the region. The six GCC states have approved the new Arab guidelines, which will be effective from July 2015 (Saad 2014).
A full understanding of the concept of effective communication can be achieved by investigating all aspects of the pharmacovigilance (PV) approach that are adopted in the Gulf region. Similarities and differences between the six systems should be determined. The PV practices should be assessed from the regulator’s and pharmaceutical industry’s perspective. This involves evaluation of the reporting practices, the causality assessment methods used in the region and the level of consistency in the information transfer of drug safety and in the decision-making process across the region. The established similarities in the key components of the regional pharmacovigilance systems determine the ability of the existing stakeholders to effectively use and/or communicate benefit-risk data. Such effective interaction should reflect successful standardisation of the PV systems in the Gulf region.
Current approaches utilised by the regulatory authorities and pharmaceutical companies in the Gulf region have been evaluated for existing PV activities that enable effective communication of risks amongst various stakeholders.
The six Gulf states (Bahrain, Kuwait, Oman, Saudi Arabia, Qatar, UAE) and a cohort of 15 pharmaceutical companies were invited to share their experiences with establishing a pharmacovigilance system.
Several reports have commented on the complexity of healthcare communication (Chused et al. 2008). However, the literature lacks references to PV and risk communication (RC) in the Gulf region. The only article found is by Wibur (2013), which has discussed the need for active PV programmes to monitor adverse drug reactions occurring in the local populations of Qatar. This study described the knowledge, experience, attitudes and perceived barriers to reporting suspected adverse drug reactions in Qatar. It evaluated the willingness of the pharmacists to engage in PV activities supported by training and transparency in the reporting process. A study by Shin et al. (2013) addressed the AEs attributed to Korean traditional medicines and provided useful information about the methods used to record AEs in Korea and how reports are missed by the Korean PV centre. Patidar et al. (2013) did another study in Mumbai that addressed best approaches for active surveillance to produce satisfactory public health decisions. These include interviewing patients and physicians and consulting medical records to produce accurate information. A further literature search produced information about the basis of the PV system in Italy (Mazzitello et al. 2013; Pimpinella and Tartaglia 2013; Giofre et al. 2013). These studies generated inputs that would be beneficial for the establishments of more efficient PV systems in the Gulf region. Finally, Palleria et al. (2013) addressed limitations and obstacles of spontaneous adverse drug reaction reporting which provided useful information for evaluating the current challenges and driving forces to achieve strong PV systems in the Gulf region.
The evaluation of the role of regulatory authorities and the pharmaceutical companies in assessing and communicating benefits and risks of medicines in the Gulf region was reviewed. It is important to note that the PV system is generally in its early stages in the GCC region. Recommendations obtained from the Kuwait Conference on National Pharmacovigilance Strategies (2014), under the patronage of Kuwait Minister of Health and the GCC Health Ministers’ Council, indicated that underreporting of adverse drug reactions and adverse drug events is a result of the lack of effective interactions and information transfer of drug safety data between the relevant stakeholders. This is mainly due to the existing culture of poor awareness about good reporting practices from the healthcare professionals’ and consumers’ perspectives. Therefore, it was important to investigate the critical aspects of the existing PV initiatives to understand the gaps that might be hindering effective drug safety information transfer across the Gulf region.
The pharmaceutical sector in the Gulf region has experienced a considerable growth over the years due to the favourable demographic and economic conditions. However, the sector is still facing some obstacles as the market is in its emerging phase, drug manufacturing is at its early steps and the six states are continuing to depend on imported medicines. The GCC governments are striving to decrease their dependence on branded pharmaceutical products by increasing local drug manufacturing through joint ventures and licensing deals with multinational pharmaceutical companies. This collaboration between the government and the pharmaceutical industry can be further extended to reach the consumer of medicines by effectively communicating benefits and risks to them.
Current Pharmacovigilance Practices in the Gulf Region
The Gulf region is considered a strategic pharmaceutical market which has developed dramatically over the past decade and placed the Gulf states in a position where establishing joint efforts became an inevitable choice to face the increasing regulatory challenges in the region. Since 2004, the Gulf states have experienced many obstacles in monitoring adverse drug reactions, their assessment and communication from the regulatory authorities and the pharmaceutical industry’s perspectives. Some Gulf states managed to initiate a well-structured system and have advanced their policies and practices to be in line with the most developed PV systems in the world, whilst others lag behind and have not been able to catch up with the increasingly growing regional or international developments. The pharmaceutical companies will not be able to effectively carry out good pharmacovigilance practices (GVPs) in the GCC region without clear and comprehensible regulations and guidelines for best PV practices according to the available resources, capacities and capabilities. Therefore, a systematic approach to investigate the current status of the existing PV strategies was carried out to provide a clear view of the significance of medicines’ risk communication in the Gulf region.
Pharmacovigilance Practices from the Regulatory Authorities’ Perspectives
Pharmacovigilance (PV) was initiated a decade ago in the Gulf region as a response to increasing scientific knowledge and awareness about the importance of monitoring the safety profile of marketed medicines. However, knowledge about the methods of detection, assessment, management and communication of risks after issuing the marketing authorisation of medicines did not emerge until recently. This was an outcome of the evolution in international regulatory systems, which aimed at ensuring the availability of quality, safe and effective medicines to patients.
The six Gulf countries were assessed for the PV activities that are carried out by the regulatory authorities at a national level (Table 6.1).
Table 6.1
Current initiative of pharmacovigilance in each Gulf states
Questions | Bahrain | KSA | Kuwait | Oman | Qatar | UAE |
|---|---|---|---|---|---|---|
Do you have a PV department? | No | Yes | Yes | Yes | No | Yes |
How many people employed in the PV department | NP | 14 | 3 | 3 | NA | 2 |
To whom does the PV department report? | NP | Vice President of Drug Affairs | Head of Registration Department who reports to the Registration and Release Superintendent | Director of Drug Control | NA | Director of Department |
Do you collaborate with other authorities within the region? | NP | No | Yes | Yes | Yes | Yes |
Do you work with the industry? | NP | Yes | Yes | Yes | Yes | Yes |
The responses revealed that two regulatory authorities (Bahrain and Qatar) have not yet established PV units, which could be due to the lack of experts and qualified personnel to establish and operate the system effectively. Another reason could be due to the lack of funding or proper strategic planning. Three authorities (Kuwait, Oman and UAE) established their PV units with no more than three employees to carry out basic PV activities such as receiving the adverse drug reaction reports and monitoring safety data received from local, regional or international sources. Saudi Arabia employs the largest number of trained personnel in the PV department and has a well-established PV system compared with other GCC states (Table 6.1).
Four regulatory authorities (Kuwait, Oman, Qatar and UAE) mentioned that they collaborate with other authorities in the region. Saudi Arabia, however, did not indicate any existing collaborative efforts with their neighbouring authorities (Fig. 6.1).
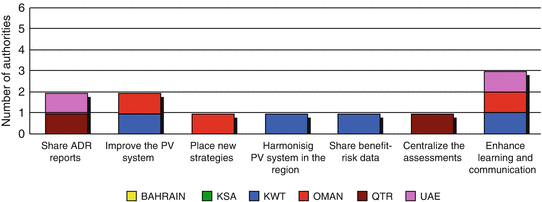

Fig. 6.1
Purpose of collaborative efforts between the GCC regulatory authorities
Kuwait was the most supportive of the six states, indicating their need to improve their PV system, to share information about the benefits and risks of medicines with the neighbouring authorities and finally to enhance the learning ability and effective communication between the GCC countries.
Furthermore, all regulatory authorities (except Bahrain) support the idea of working with the PI but indicated various answers when presented with five possible choices (Fig. 6.2). The most common reason for working with the PI was sharing information about any developments in the safety and efficacy profiles of the marketed medicines. This was indicated by four regulatory authorities (Saudi Arabia, Oman, Qatar and UAE). Three authorities support the need to work with the PI to improve the current system, to improve the adverse drug reaction reporting and the communication and transparency between the two stakeholders. Saudi Arabia supports all the reasons provided. Only Kuwait and Saudi Arabia support the need to work with the PI to initiate the PV system.
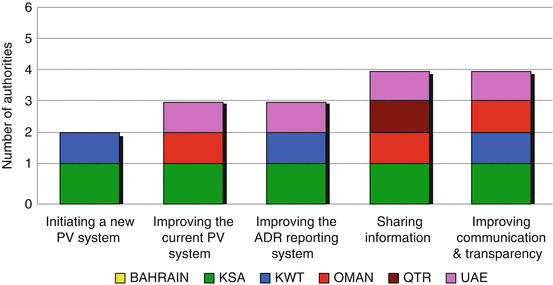

Fig. 6.2
Reasons for working with the pharmaceutical industry
Sources of Adverse Drug Reaction Reports
Two methods were indicated by five authorities’ which included submission of adverse drug reaction reports by the pharmaceutical companies and spontaneous reporting (Fig. 6.3). Saudi Arabia, Oman and UAE support all methods whilst Kuwait added another method, which is the use of reference agencies to obtain information about newly reported adverse drug reactions. UAE, however, uses direct patient complaint reports in addition to the stated methods.
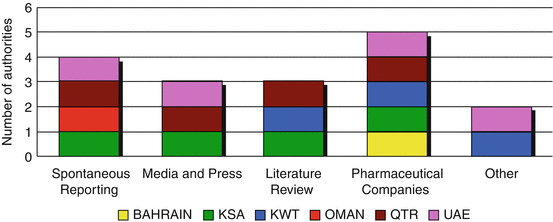

Fig. 6.3
Methodology for collecting adverse drug reaction reports by the GCC regulatory authorities
The groups that most commonly reported adverse drug reactions were the pharmaceutical companies, physicians/dentists and pharmacists (Fig. 6.4). Only three authorities stated the patients and consumers as sources of adverse drug reaction reports. Saudi Arabia and UAE support all types of reporting sources.
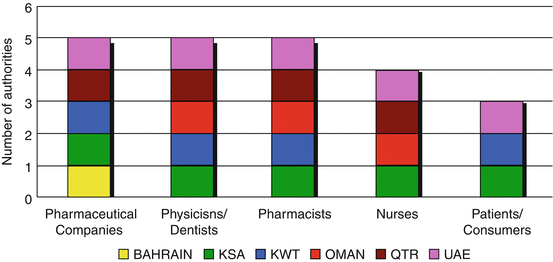

Fig. 6.4
Adverse drug reaction reporters to the GCC regulatory authorities
Monitoring of Adverse Drug Reaction Reports
Five authorities (Saudi Arabia, Kuwait, Oman, Qatar and UAE) have standard adverse drug reaction reporting forms. Internal assessors in Saudi Arabia, Kuwait and Oman evaluate the completed forms submitted by the reporting personnel. Qatar appoints a committee to evaluate the adverse drug reaction reporting form, whilst UAE uses internal assessors who present the conclusion to the committee to make the appropriate decision. However, for such monitoring to be effective, these five GCC states believe that the industry have a mandatory role in communicating all the safety information and reporting the adverse drug reactions to the national regulatory authorities (Table 6.2).
Table 6.2
Management of the adverse drug reaction reporting system in each Gulf state
Question | Bahrain | KSA | Kuwait | Oman | Qatar | UAE |
|---|---|---|---|---|---|---|
Do you have a standard reporting form? | No | Yes | Yes | Yes | Yes | Yes |
Who evaluates adverse drug reaction reports? | NP | Internal Assessor | Internal Assessor | Internal Assessor | Committee | Internal Assessor |
Committee | ||||||
Authorities views on the role of industry in reporting adverse drug reactions? | NP | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory |
The next step in the process of monitoring the adverse drug reaction reports is to identify the type of methods used to assess the possible causes of the adverse events. Three authorities (Saudi Arabia, Oman and UAE) use algorithms and expert judgments to assess the causal relationship between the medicine and the adverse event. Kuwait depends on expert judgment whilst Qatar indicated that they use case-controlled studies for the purpose of assessing the cause of the adverse event (Fig. 6.5).
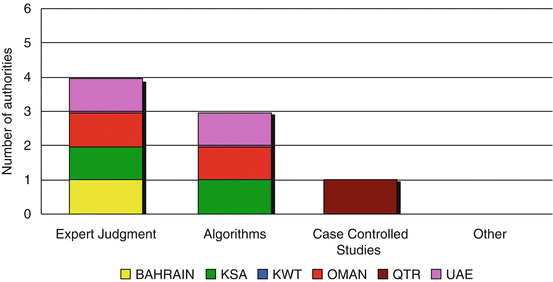

Fig. 6.5
Causality assessment methods adopted in the Gulf region
Benefit-Risk Assessment and Communication
The concept of evaluating the risks of medicines in the light of their benefits has recently been recognised as an important factor that affect the decision-making process in relation to determining a positive benefit-to-risk ratio. All the Gulf states indicated their awareness about the periodic benefit-risk evaluation reports that replaces the PSURs. Five states (all except Bahrain) support the benefit-risk assessment after granting the marketing authorisation of medicines. They also support the need for having a well-structured framework for assessing the benefits and risks of medicines during the pre-marketing review and have established a mechanism for communicating the outcomes of the adverse drug reaction reports. However, only UAE has a structured framework for the benefit-risk assessment during the review process, which follows the EMA’s format. Four authorities (Saudi Arabia, Kuwait, Oman and Qatar) have plans to initiate such a framework and would be interested in receiving appropriate recommendation for its implementation (Table 6.3).
Table 6.3
Status of benefit-risk assessment and communication in the Gulf
Question | Bahrain | KSA | Kuwait | Oman | Qatar | UAE |
|---|---|---|---|---|---|---|
Are you aware of the PBRER? | Yes | Yes | Yes | Yes | Yes | Yes |
Is it of value to review adverse drug reactions without looking at the benefits of the medicines? | NP | No | No | No | No | No |
Should there be benefit-risk assessment in the post-marketing period of medicines? | NP | Yes | Yes | Yes | Yes | Yes |
Do you have a benefit-risk assessment framework for the regulatory review phase? | NP | No | No | No | No | Yes as per EMA |
Would you be interested in being provided with a framework? | NP | Yes | Yes | Yes | Yes | NA |
Do you have a plan to initiate a framework for benefit-risk assessment? | NP | Yesa | No | Yes | No | NA |
Do you have an established mechanism for communicating the outcome of the reported adverse drug reactions? | NP | Yes | Yes | Yes | Yes | Yes |



