(1)
University of South Australia, Adelaide, Australia
Abstract
The term “innovation” is used in a number of different ways in pharmaceutical regulation processes. In this chapter I address the question of what is meant by the term “innovation” in the context of the debate on new drug prices. I distinguish between lay and medical uses of the terms “innovation” and “innovative” then I identify three ways that pharmaceutical innovation generates a social surplus. First, clinical innovation, which is the “incremental effect” used in cost-effectiveness analysis and quantified for a specific clinical context and patient group. Resource innovation is the second source of surplus: innovation in the resources involved in supplying a given clinical benefit, for example, an oral version of an intravenous drug. Third, developing and manufacturing innovation, for example, innovation in the methods of manufacturing drugs. An analogy between clinical and economic concepts of value is noted. Clinical value of innovation, like economic value, is constrained by the best alternative strategy. The clinical value of a new drug’s innovation is its gross clinical effect (compared with no care) constrained by the opportunity cost (foregone health benefit) to the patient of not using the best existing therapy.
4.1 The Reimburser’s Problem
The US International Trade Delegate is arguing that pharmaceutical regulation needs to “reward innovation” and that the pricing system in the country of interest does not achieve this. The Reimburser is aware that a recent decision about a new drug was controversial because the Reimburser refused to pay more for that drug (on a cost per course basis) compared with a drug that had been off-patent for at least 5 years (a generic). The reason for her decision was that the new drug was shown in clinical trials to be no more effective than the existing generic drug. The new drug was innovative in that it had a different molecular structure from any existing drug, and it was even the lead drug in a new therapeutic sub-class1; however, the new drug did not provide a clinical advantage over the standard therapy for this condition. The International Trade Delegate argues that a significant investment was made into researching and developing this innovative drug and the higher price was needed to reward this investment in innovation. The International Trade Delegate points out that the firm that took this risk and invested would have been better off if it had simply produced the generic. Where is the incentive for innovative firms to take risks if they are rewarded no more than generic firms that take no risks? He argues that it does not make economic sense for this investment in the innovation process to remain unrewarded.
The Reimburser is concerned about the concepts of innovation revealed by the discussion with the US International Trade Delegate. From a clinical perspective, the value of innovation is about the clinical benefit of the new drug, not about the characteristics of the molecule or the type of risks taken by the firm. However, the Health Economic Adviser points out that clinical innovation is not necessary for there to be pharmaceutical innovation. A new drug could be no more effective than an existing drug but delivered in a way that does not require a hospital admission, hence reducing the cost associated with administering the drug. Hence the innovation in this case is not about clinical benefit; it is about resource benefit.
Is there a fundamental difference between how economists and clinicians understand the value of pharmaceutical innovation? The Reimburser asks her Health Economic Adviser:
Is there a way of defining the value of pharmaceutical innovation that makes sense from both an economic and clinical perspective?
4.2 Innovation: Lay, Regulatory and Medical Concepts
4.2.1 Innovation and the Regulatory Process
The term “innovative” is used to distinguish between generic firms (that do not invest in pharmaceutical R&D and only produce generic drugs) and innovative firms (that do invest in pharmaceutical R&D).2 If an innovative firm develops a new molecule, it only needs to establish a sufficient degree of physical difference from an existing technology to be defined as an “innovation” or “invention”. For example, a New Molecular Entity (NME) can be awarded a patent provided it is different from any existing molecule. Whether it is more or less effective than a placebo is not relevant to the decision to define it as “innovative” or an “invention” for the purpose of a patent. Consequently, each year there are many more molecules patented and tested in phase 1 trials than there are NMEs registered by the FDA (approved for therapeutic use).3 , 4
For a firm to be provided with a licence to market that drug, it requires evidence of the clinical value of this innovative molecule, typically evidence of its safety and efficacy.5 In most jurisdictions, the minimum evidence of clinical value of innovation is obtained by controlling (constraining) the effect of the new drug by some alternative therapy. For the firm to obtain a licence to market a new drug in the US it requires evidence of its performance against placebo, preferably derived from a double-blind randomised controlled trial (RCT). The evidentiary and performance demands on a licenced drug (one that is approved by an agency such as the FDA in addition to being patented) are therefore much higher than those on an innovative molecule (one that is awarded a patent). Some new drugs that are approved by the FDA are referred to as “innovative” to distinguish between first-in-class (innovative or lead) and follow-on (“me-too”) drugs (Pekarsky 2010). However, not all drugs that are approved for use or described as “innovative” necessarily have a clinical value in the underlying innovation. So what is clinical innovation?
4.2.2 Clinical Innovation
HTA/CEA informs the so-called “fourth hurdle” of regulation where the quantification of the new drug’s clinical innovation is the primary objective of the analysis.6 The regulatory imperative of reimbursement decisions is to assess the appropriateness of changed, additional or substituted therapy. Therefore, reimbursing institutions are interested in the clinical value of innovation for a group of patients for whom a subsidy for the cost of the drug is being proposed. A common definition of the clinical value of innovation of a new technology is the best estimate of the additional clinical effect (or effects) of the new technology compared to the best existing therapy for the group of patients for whom its use is being assessed.7 , 8 This definition requires specificity in the assessment of the “clinical value of innovation” of a new technology. For example, an HTA/CEA assessor is unlikely to ask the question: “Is this new drug clinically innovative?” Instead he might ask: “If current best therapy is replaced by this new drug for this patient group, using this clinical protocol (tests, dose and duration of therapy) what is the expected incremental effect as measured by this set of clinical endpoints?”
HTA/CEA typically involves meta-analyses of the evidence of effect from RCTs and the extrapolation of this evidence to longer time periods, additional patient groups and clinical endpoints by way of pharmaco-economic models. Uncertainty is characterised and analysed using deterministic and probabilistic sensitivity analyses. The net financial implication to the health budget of adoption, ΔC, is also estimated. (See the discussion about resource innovation in Sect. 4.3.) The incremental financial cost, ΔC, and incremental effect, ΔE, of the new drug compared with the best alternative therapy are summarised as either an incremental cost-effectiveness ratio:
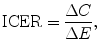
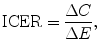
or a net benefit (NB) metric:
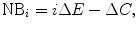
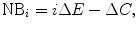
where  is some monetary value of the clinical effect.9 [See Pekarsky (2012, Appendix 4) for a discussion of this terminology.]
is some monetary value of the clinical effect.9 [See Pekarsky (2012, Appendix 4) for a discussion of this terminology.]
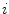 is some monetary value of the clinical effect.9 [See Pekarsky (2012, Appendix 4) for a discussion of this terminology.]
is some monetary value of the clinical effect.9 [See Pekarsky (2012, Appendix 4) for a discussion of this terminology.]This information is then used in conjunction with a range of other evidence to inform the decision to reimburse that drug.10 While HTA/CEA methods vary across jurisdictions, there is a key common element; the focus on the estimate of ΔE for a specific clinical context (comparator, patient group and clinical protocol). A drug might be described generally as clinically innovative, but the estimate of value of clinical innovation is specific to a clinical context.
4.3 Non-clinical Pharmaceutical Innovation
The focus on clinical innovation (ΔE) as the tangible (and valuable) outcome of pharmaceutical innovation is consistent with the narrative around medical research more generally: it is about developing cures and treatment for diseases and improving life expectancy and quality of life for patients.11 However, pharmaceutical innovation (the product of pharmaceutical R&D) can take at least two other forms and still potentially impact on population health, without having any clinical innovation content in the new drug. The first form is in relation to the implications for health resource use generally and the second relates to innovation in the drug manufacturing process.
4.3.1 Resource Innovation
The incremental impact of a new drug on resource use is:
The conventional ΔC (the net financial impact of adopting the new drug compared to existing therapy);
Less the share of that additional financial cost that is attributed to either:
The financial cost of the new drug less the financial cost of the drug that it substitutes for; or
The financial cost of the new drug if it is added to existing therapy (no substitution).
We start with an example of pure resource innovation: a new formulation of a drug that allows the drug to be taken orally at home rather than intravenously as part of a hospital admission. This innovation could result in a reduction in the non-drug costs of $250 per course of the drug. In this case, the difference in resource use is captured in the ICER or NBi via ΔC. From an economic perspective, this innovation can be valued in terms of increased population health, for example if the additional financial savings are allocated to other health services. However, if the firm prices the new drug so as the additional savings are entirely offset by the additional cost of the drug, then the entire value of the resource innovation is appropriated by the firm.12 For this reason, ΔC only captures “resource innovation” if the net additional cost of the new drug relative to the existing substituted drug is excluded.13 , 14
4.3.2 Manufacturing Innovation
Another form of non-clinical innovation is in the manufacturing process. Typically the variable costs of producing drugs is argued to be low relative to the cost of R&D; however, there remains scope to reduce the manufacturing costs.15 , 16 Reduced cost of manufacturing is the source of innovation used by Tirole (1988) to illustrate the “pure value of innovation.” Tirole shows how if the firm is a monopolist in its output market it can maintain a price per unit of the good following innovation in manufacturing and the entire surplus is appropriated by the firm. In more competitive situations financial savings will be shared with purchasers. This example of innovation in manufacturing is expanded in Chap. 10 in terms of its implications for the pricing of drugs today in order to gain innovation in the future.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


