, Mohammed Al-Rubaie2, Stuart Walker3 and Sam Salek4
(1)
Regulatory Affairs Consultant Executive Directive, Kuwait Advancement for Conference and Exhibition Management, Kuwait, Kuwait
(2)
Ministry of Health, Directorate General of Pharmaceutical Affairs and Drug Control, Muscat, Oman
(3)
Founder of Centre for Innovation in Regulatory Science, London, UK
(4)
Department of Pharmacy School Life & Medical Sciences, University of Hertfordshire, Hatfield, UK
Introduction
The approval of medicines in different countries across the world has been and still is performed by regulatory agencies according to their national regulations, although attempts to harmonise the regulations have been made for several years within the GCC region. The harmonisation of the regulatory review processes in the GCC states was initiated following the issuance of the GCC Health Ministers’ Council Decree No. 8 in 1976 regarding the formation of a study group to report on how a centralised registration review system should be established to monitor the marketing of medicines and develop common guidelines for the participating authorities (Al-Essa 2011).
This was followed by a series of GCC Ministerial Decrees relating to the establishment of a centralised registration system which was not approved until the Kingdom of Bahrain submitted a proposal for the formation of a ‘Central Committee for the Gulf States’ to register pharmaceutical companies and their products. This committee ensures that the pharmaceutical companies apply satisfactory standards to guarantee manufacturing of high-quality, safe and effective medicines and to standardise their regulations with regard to medicines importation practices in the Gulf states. The Centralised Procedure for the registration of pharmaceutical companies and medicinal products has been established under the Executive Board of the Health Ministers’ Council for GCC states. The Executive Board is chaired by the Director General, who is responsible for supervising the work of the board and following up the resolutions and recommendations of the Health Ministers’ Council, assisted by technical, administrative and financial departments.
The Gulf Centralised Committee for Drug Registration (GCC-DR) was formed in May 1999 and included Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, the United Arab Emirates and Yemen (joining in 2004). The GCC-DR consists of two representatives from each member state. The main role of the committee is to register pharmaceutical companies and their products through the joint coordination of the safety, efficacy and quality of medicinal products.
In order to adopt the central registration system, the following two phases were proposed:
Phase I: In 2000, pharmaceutical companies in the Gulf states had to register their products using the local system whilst at the same time it was mandated that they also use the centralised system. During a period of 2 years, the following was required:
The registration of research companies by the GCC-DR in accordance with the centralised registration.
The registration of new chemical entities and biological and new technology products.
A priority was given to the Gulf states’ companies to register centrally.
The registration of products, which have not been through the Centralised Procedure but have been selected for the GCC international tender.
Phase II: The second phase took effect in 2002 following an evaluation of phase I, and during this phase, all the companies could submit their products through the GCC procedure.
During the 11 years (1999–2010), the GCC centralised system received 1,824 medicinal product applications and approved 1,165. This study has highlighted the different steps in the review process in the GCC-DR system and the way in which these influenced the overall timelines. Information was obtained to identify the practices that accelerate or delay the marketing authorisation. Therefore, this study mapped the key milestones and associated activities and evaluated the quality measures employed by the GCC-DR process.
Information on the total number of applications and approvals together with the timelines from January 2006 to December 2010 were obtained directly from the Executive Board of the Health Ministers’ Council for GCC states. The data included the application submission date, the registration date and the overall review time. The products were classified into four groups according to the location of the manufacturing companies (GCC, non-GCC Arab, international and Asian). The data also included information on different dosage forms (solid, semi-solid, liquids, injectable) and the therapeutic indications.
Currently the Executive Board is responsible for receiving the registration files and the prescribed fee for the registration of pharmaceutical manufacturing companies and their products. After receiving the files, the Executive Board forwards the files to all the member states for their evaluation. Every member state should review the files and provide their recommendations to the GCC-DR. The registration of the company is decided based on the recommendations issued by of the good manufacturing practice (GMP) inspection team. If the GMP team recommends the registration of the company, the GCC-DR issues the registration certificate; otherwise the company will be notified accordingly. The inspection team consists of three members from three different member states. The selection of members is carried out based on certain criteria where every member state would have an equal number of visits. Upon completion of the visit, the head of the inspection team will prepare a visit report, reviewed and signed by other members of the team. The report is, then, submitted to the GCC-DR. Similarly, the GCC-DR forwards the registration files of the products to each individual member state for evaluation. The samples are sent for analysis to one of the four laboratories (Saudi Arabia, UAE, Kuwait and Oman), which are accredited by GCC-DR. The registration of products is determined on the basis of the analysis reports.
There are basically five steps for the regulatory authority in the Gulf central registration system. Some of these steps are critical and constitute a substantial part of the review process (Fig. 7.1). The review process map indicates the main steps in the review and approval process and identifies the key milestones for monitoring and analysing timelines. Receipt and validation include administrative registration (reference number) and checks on legal requirements, the status of the company, manufacturer, etc., as well as a ‘checklist’ validation of the application content (e.g. technical sections, CPP status).
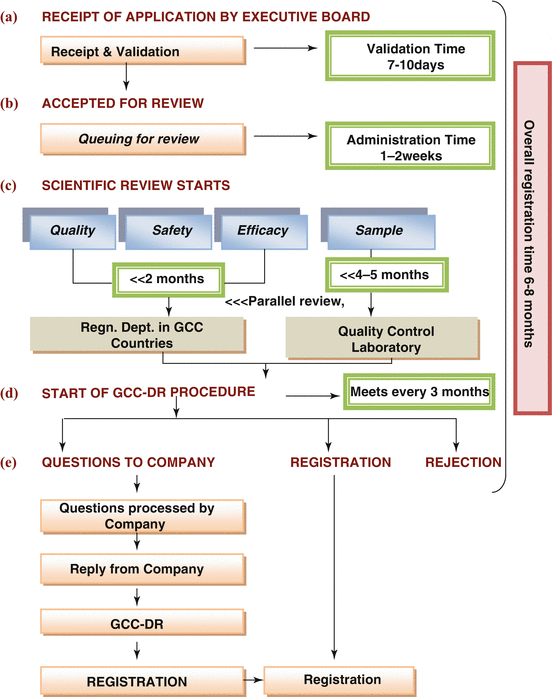

Fig. 7.1
Process map for GCC centralised registration
The pharmaceutical companies are required to submit 16 samples of their product in addition to eight copies of the product dossier and one original copy, which would remain with the Gulf Central Registration Office. While two separate GCC states would be chosen through a computer program alphabetically according to the countries’ name, to act as rapporteurs, the other GCC states would keep the dossiers for documentation purposes. The rapporteurs are not chosen because of their expertise in a certain area of regulatory science, but to give equal opportunities to all the member states in a systematic manner of selection. Similarly, the reference laboratory chosen, to carry out the analysis, is based on the equity of the number of dossiers distributed between these laboratories and not on the ability of these laboratories to perform the analysis.
Products Considered and Their Characteristics
During the 5-year period (2006–2010), the number of pharmaceutical products for human use successfully registered through the GCC centralised registration procedure was 413. For the purpose of clarity, the information provided by the GCC Centralised Procedure office in Riyadh are presented under four headings:
Number of products approved in the Gulf Centralised Procedure
Approval time in the Gulf Centralised Procedure
Approval time for different dosage forms
Approval time for different therapeutic groups
Number of Products Approved in the Gulf Centralised Procedure (2006–2010)
A total of 413 pharmaceutical products (EASs and NASs) were registered by GCC-DR during the time period 2006–2010 (Fig. 7.2).
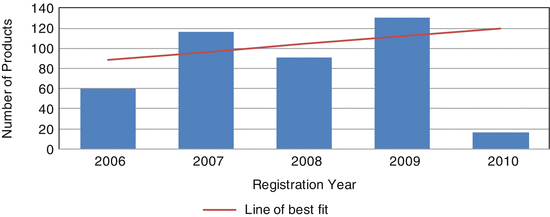

Fig. 7.2
Total number of pharmaceutical products approved in the Gulf Centralised Procedure (2006–2010)
There was a substantial drop in the number of approved products in 2010 which was due to the fact that the pharmaceutical companies failed to comply with the new GCC guidelines for the stability testing of active pharmaceutical ingredients (APIs) and finished pharmaceutical products (FPPs). The GCC countries come under climatic zones III and IVa (hot and dry and hot and humid); the new guideline which was implemented in 2009 replaced the long-term testing condition of 25 °C ± 2 °C/60 % R ± 5 % RH for climatic zones III and IV with 30 °C ± 2 °C/65 % RH ± 5 % RH. The results showed an overall significant association (p < 0.001) between the total number of registered pharmaceutical products and registration years.
Out of the total of 413 products approved in the Gulf central registration between 2006 and 2010, 317 products were EASs (Table 7.1) and 96 NASs (Table 7.2).
Table 7.1
Number of EASs approved in the Gulf Centralised Procedure from various regions, dosage forms and therapeutic classes
Characteristics | Registration year | |||||
|---|---|---|---|---|---|---|
2006 | 2007 | 2008 | 2009 | 2010 | ||
Region | Number of EASs | Total | ||||
Arab | 7 | 9 | 12 | 20 | 3 | 51 |
Asian | 6 | 2 | 7 | 3 | 0 | 18 |
Gulf | 38 | 64 | 46 | 46 | 12 | 206 |
International | 5 | 10 | 11 | 16 | 0 | 42 |
Dosage form | ||||||
Injectables | 16 | 4 | 14 | 9 | 0 | 43 |
Liquids | 6 | 18 | 15 | 22 | 3 | 64 |
Semi-solid | 9 | 15 | 13 | 6 | 1 | 44 |
Solid | 25 | 48 | 34 | 48 | 11 | 166 |
Therapeutic class | ||||||
Anaesthesia | 2 | 0 | 0 | 1 | 0 | 3 |
Cardiovascular system | 14 | 8 | 20 | 15 | 1 | 58 |
Central nervous system | 4 | 8 | 9 | 9 | 3 | 33 |
Ear, nose and oropharynx | 0 | 1 | 0 | 0
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| ||